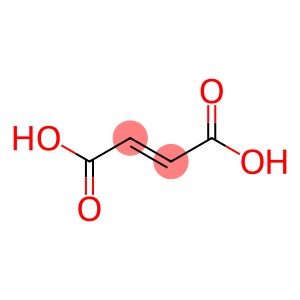
Fumaric acid CAS 110-17-8
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36 – Irritating to the eyes |
| Safety Description | 26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 9126 |
| WGK Germany | 1 |
| RTECS | LS9625000 |
| TSCA | Yes |
| HS Code | 29171900 |
| Toxicity | LD50 orally in Rabbit: 9300 mg/kg LD50 dermal Rabbit 20000 mg/kg |
Introduction
Fumaric acid. The following is an introduction to the properties, uses, preparation methods and safety information of transbutalic acid:
Quality:
- Transbutadiic acid is a colorless crystal or white solid with a pungent sour taste.
- It is soluble in water and many organic solvents such as alcohols and ethers.
- At high temperatures, transbutylic acid breaks down to produce carbon dioxide and acetone.
Use:
- It is widely used in the preparation of polyester resins for the manufacture of products such as coatings, plastics, and fibers.
Method:
- Transbutenedic acid can be obtained by the reaction of brominated butene and sodium carbonate. The specific synthesis method involves multiple steps, including the preparation of butene, bromination reaction, and alkaline hydrolysis.
Safety Information:
- Transbutadiic acid is an irritating compound that may cause irritation and burns in contact with the skin and eyes.
- Wear appropriate personal protective equipment such as gloves, glasses, and protective clothing during handling.
- Avoid inhaling its dust or vapours and should operate in a well-ventilated area.
- Local regulations and safe operating procedures should be followed when storing and handling the compound.