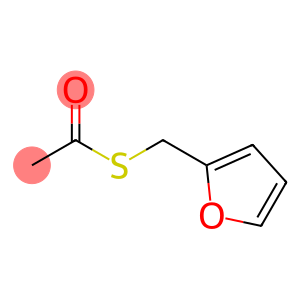
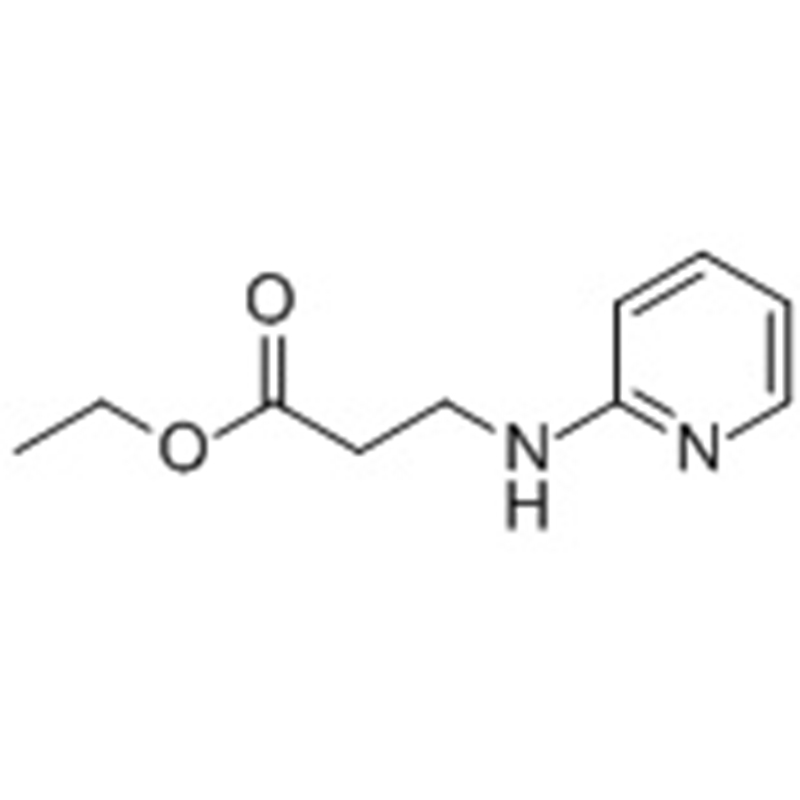
Fufuryl thioacetate(CAS#13678-68-7)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 3334 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29321900 |
| Hazard Class | 9 |
Introduction
Methyl thioethyl S-acid branyl. The following is an introduction to the properties, uses, preparation methods and safety information of methyl thioethyl thioS-acid furfur:
Quality:
Methyl thioethyl S-fufrate is a colorless liquid with a special methyl sulfate taste. It is soluble in organic solvents such as alcohols, ketones, and ethers, but insoluble in water.
Use:
Methyl thioethyl S-furfur is widely used in the field of organic synthesis. It can be used as a reagent for various organic synthesis reactions, such as the carbonylation of amines, the esterification and acylation of alcohols, etc. Methyl thioethyl S-furfur can also be used as a preservative and solvent.
Method:
The preparation of methyl thioethyl S-acid furfur is usually obtained by the reaction of carbon disulfide with methyl chloroacetate. The specific preparation method is as follows: carbon disulfide is slowly dropped into methyl chloroacetate in ice water, and the reaction solution is stirred at the same time. After the reaction is completed, the reaction solution is added to the saturated sodium chloride solution, and then the organic layer is extracted and dried with anhydrous sodium chloride. Methyl thioethyl S-acid furfur is obtained by distillation.
Safety Information:
Methyl thioethyl S-furfur is an organic solvent with a pungent odor and should be avoided when used. When operating, appropriate protective equipment such as protective glasses and gloves should be worn. It should be stored in a cool, dry, well-ventilated place, away from ignition and oxidizers, to avoid fire and explosion. For the use and storage of any chemicals, it is important to follow proper safe handling practices.