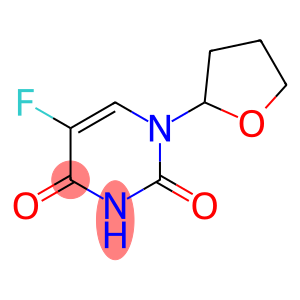
Ftorafur(CAS#17902-23-7)
| Hazard Symbols | T – Toxic |
| Risk Codes | 23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. |
| Safety Description | S22 – Do not breathe dust. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | YR0450000 |
| HS Code | 29349990 |
| Hazard Class | 6.1 |
| Packing Group | II |
| Toxicity | LD50 in mice (mg/kg): 900 orally (3 days) (Yasumoto); 750 i.p. (FR 1574684), also reported as 1150 i.p. (Smart) |
Introduction
Trifluoromethylation is an organic chemical reaction in which trifluoromethyl groups can be introduced into organic molecules using tegafluor reagents such as TMSCF3.
Properties of tegafluor:
- Tegafluor is an important group conversion reaction, which can introduce trifluoromethyl groups with a certain electron density to change the physical and chemical properties of molecules.
- Trifluoromethyl groups have strong electron attraction, which can increase the electrophilicity of the molecule and the solubility of the solvent.
- The products of the tegafluor reaction are generally chemically stable and biologically active.
Uses of tegafluor:
- In the field of materials science, tegafluor can change the surface properties of materials, increase their stability and weather resistance.
Preparation method of tegafluor:
- Commonly used tegafluor reagents include: TMSCF3, Ruppert-Prakash reagent, etc.
- Tegafluor reactions are usually carried out in an inert atmosphere, using an inert solvent (e.g., methylene chloride, chloroform) as the reaction medium.
- Reaction conditions generally require higher reaction temperatures and longer reaction times, and often require the addition of a catalyst (e.g., copper catalyst).
Safety information on tegafur:
- Tegafluor reagents are toxic and corrosive, and appropriate precautions need to be taken when handling.
- Gases (e.g. hydrogen fluoride) produced during the reaction are also dangerous and need to be operated under well-ventilated conditions.
- Care should be taken to avoid contact with water or humidity during operation to avoid irreversible chemical reactions.
- Reactants and products under tegafluor reaction conditions require proper treatment and waste disposal.