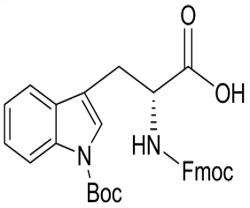
Fmoc-D-Trp(Boc)-OH(CAS# 163619-04-3)
Risk and Safety
| Safety Description | S22 – Do not breathe dust. S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 10 |
| HS Code | 29339900 |
Fmoc-D-Trp(Boc)-OH(CAS# 163619-04-3) introduction
N-alpha-fluorene methoxycarbonyl-N-in-tert-butoxycarbonyl-D-tryptophan is an amino acid derivative, also known as Fmoc-Trp(Boc)-OH. Here is some information about its properties, uses, manufacturing methods, and safety:
Quality:
- Appearance: White crystalline solid
- Solubility: Soluble in organic solvents such as methylene chloride and dimethyl sulfoxide, insoluble in water
Use:
- Fmoc-Trp(Boc)-OH is widely used in the field of peptide synthesis and is used as a protective group in organic synthesis.
Method:
- The preparation of Fmoc-Trp(Boc)-OH typically consists of two steps. The amino groups of tryptophan side chains are protected with a protecting group, usually with dihydrazine spinachlate (Fmoc). Second, tert-butylhydroxymethylic acid acetal (Boc) is used to protect the hydroxyl group of tryptophan.
Safety Information:
- Fmoc-TRP (Boc)-OH may be irritating to the skin, eyes, and respiratory system, and should be used with appropriate protective equipment to ensure adequate ventilation.
- Care should be taken to avoid inhalation, swallowing, or contact when using or handling Fmoc-Trp(Boc)-OH.