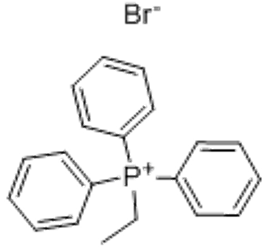
(Ethyl)triphenylphosphonium bromide (CAS# 1530-32-1)
Risk and Safety
| Risk Codes | R22 – Harmful if swallowed R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R36/37/38 – Irritating to eyes, respiratory system and skin. R21/22 – Harmful in contact with skin and if swallowed. |
| Safety Description | S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| UN IDs | UN 3077 9/PG 3 |
| WGK Germany | 2 |
| TSCA | Yes |
| HS Code | 29310095 |
| Hazard Class | 6.1 |
| Packing Group | III |
Reference Information
| LogP | -0.69–0.446 at 35℃ |
| EPA chemical information | Information provided by: ofmpub.epa.gov (external link) |
| Use | Ethyltriphenylphosphine bromide is used as wittig reagent. Ethyltriphenylphosphine bromide and other phosphine salts have antiviral activity. for organic synthesis |
| preservation conditions | preservation conditions of ethyltriphenylphosphine bromide: avoiding moisture, light and high temperature. |
Introduction
Ethyltriphenylphosphine bromide, also known as Ph₃PCH₂CH₂CH₃, is an organophosphorus compound. The following is an introduction to the properties, uses, preparation methods and safety information of ethyltriphenylphosphine bromide:
Quality:
Ethyltriphenylphosphine bromide is a colorless to light yellow crystal or liquid with a strong benzene aroma. It is soluble in organic solvents such as ethers and hydrocarbons at room temperature. It has a lower solubility than water.
Use:
Ethyltriphenylphosphine bromide has a wide range of applications in organic synthesis. It acts as a phosphorus reagent for nucleophilic substitution of halogen atoms and nucleophilic addition reactions of carbonyl compounds. It can also be used as a ligand for organometallic chemistry and transition metal-catalyzed reactions.
Method:
Ethyltriphenylphosphine bromide can be prepared by the following reactions:
Ph₃P + BrCH₂CH₂CH₃ → Ph₃PCH₂CH₂CH₃ + HBr
Safety Information:
Ethyltriphenylphosphine bromide has a lower toxicity but should still be used with caution. Exposure to ethyltriphenylphosphine bromide may cause irritation and eye damage. Appropriate precautions, such as wearing gloves and goggles, should be taken when in use, and good ventilation should be ensured. Avoid inhaling its vapors or coming into contact with the skin and eyes during the operation.


![5-[[(2-Aminoethyl)thio]methyl]-N N-dimethyl-2-furfurylamine(CAS# 66356-53-4)](https://www.xinchem.com/uploads/52AminoethylthiomethylNNdimethyl2furfurylamine.png)