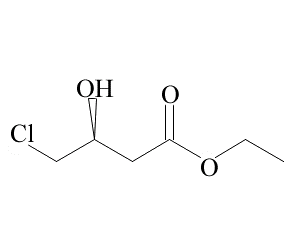
Ethyl S-4-chloro-3-hydroxybutyrate(CAS# 86728-85-0)
| Risk Codes | R41 – Risk of serious damage to eyes R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S39 – Wear eye / face protection. |
| UN IDs | 2810 |
| WGK Germany | 3 |
| HS Code | 29181990 |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
Ethyl (S)-(-)-4-chloro-3-hydroxybutyrate is an organic compound. Its main properties are as follows:
Appearance: It is a colorless liquid.
Solubility: It can be dissolved in many organic solvents such as chloroform, ethanol, and ether.
The main uses of ethyl (S)-(-)-4-chloro-3-hydroxybutyrate are as follows:
2. Organic synthesis: It can be used as a substrate or ligand for chiral catalysts to participate in various organic reactions.
Chemical research: It is commonly used in the synthesis, separation, and purification of chiral compounds.
A common method for the preparation of ethyl (S)-(-)-4-chloro-3-hydroxybutyrate is obtained by reaction of 4-chloro-3-hydroxybutyrate with glycolylation.
Appropriate protective equipment such as chemical goggles, gloves and lab coats should be worn during operation.
Avoid contact with skin, eyes, and mucous membranes.
Make sure to operate in a well-ventilated area to avoid inhaling harmful gases.
When storing, avoid contact with strong oxidants and strong acids.