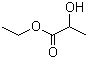
Ethyl lactate(CAS#97-64-3)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R10 – Flammable R37 – Irritating to the respiratory system R41 – Risk of serious damage to eyes |
| Safety Description | S24 – Avoid contact with skin. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S39 – Wear eye / face protection. |
| UN IDs | 1192 |
| WGK Germany | 1 |
| RTECS | OD5075000 |
| HS Code | 29181100 |
| Hazard Class | 3.2 |
| Packing Group | III |
Introduction
Lactic acid ethyl ester is an organic compound.
Ethyl lactate is a colorless liquid with an alcoholic fruity flavor at room temperature. It is soluble in organic solvents such as alcohols, ethers, and aldehydes, and can react with water to form lactic acid.
Ethyl lactate has a variety of uses. In the spice industry, it is often used as an ingredient in the preparation of fruit flavors. Secondly, in organic synthesis, ethyl lactate can be used as a solvent, catalyst, and intermediate.
There are two main methods for the preparation of ethyl lactate. One is to react lactic acid with ethanol and undergo esterification reaction to produce ethyl lactate. The other is to react lactic acid with acetic anhydride to obtain ethyl lactate. Both methods require the presence of a catalyst such as sulfuric acid or sulfate anhydride.
Ethyl lactate is a low-toxicity compound, but there are still some safety precautions to be taken into account. Exposure to ethyl lactate may cause eye and skin irritation, and appropriate protective equipment should be worn when using it. Avoid exposure to open flames and high temperatures to prevent combustion or explosion. When using or storing ethyl lactate, care should be taken to keep it away from flammable substances and oxidizing agents. If ethyl lactate is ingested or inhaled, seek medical attention immediately.