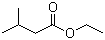Ethyl isovalerate(CAS#108-64-5)
| Risk Codes | 10 – Flammable |
| Safety Description | 16 – Keep away from sources of ignition. |
| UN IDs | UN 3272 3/PG 3 |
| WGK Germany | 2 |
| RTECS | NY1504000 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 29156000 |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Ethyl isovalerate, also known as isoamyl acetate, is an organic compound.
Quality:
- Appearance: Colorless liquid
- Smell: Has a fruity aroma
- Solubility: Soluble in ethanol, ethyl acetate and ether, insoluble in water.
Use:
- As a solvent: Due to its good solubility, ethyl isovalerate is often used as a solvent in organic synthesis reactions, especially when water-sensitive reactions are involved.
- Chemical reagents: Ethyl isovalerate can also be used as a reagent in some laboratory studies.
Method:
Ethyl isovalerate can be prepared by the reaction of isovaleric acid and ethanol. During the reaction, isovaleric acid and ethanol undergo esterification reaction under a certain temperature and catalyst to form ethyl isovalerate.
Safety Information:
- Ethyl isovalerate is somewhat volatile, and contact with heat sources or open flames can easily cause fires, so it should be kept away from fire sources.
- Airborne ethyl isovalerate vapor can cause eye and respiratory irritation, so wear protective glasses and a protective mask if necessary.
- Avoid contact with the skin to avoid skin irritation or allergic reactions.
- If ethyl isovalerate is ingested or inhaled by mistake, seek medical attention immediately.