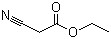
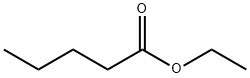
Ethyl cyanoacetate(CAS#105-56-6)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R36/38 – Irritating to eyes and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | UN 2666 |
Ethyl cyanoacetate(CAS#105-56-6) Introduction
Ethyl cyanoacetate, CAS number 105-56-6, is an important organic chemical raw material.
Structurally, it contains a cyano group (-CN) and an ethyl ester group (-COOCH₂CH₃) in its molecule, and this combination of structures makes it chemically diverse. In terms of physical properties, it is generally a colorless to light yellow liquid with a special odor, a melting point of about -22.5 °C, a boiling point in the range of 206 – 208 °C, soluble in organic solvents such as alcohols and ethers, and a certain solubility in water but relatively small.
In terms of chemical properties, the strong polarity of the cyano group and the esterification characteristics of the ethyl ester group determine that it can undergo many reactions. For example, it is a classical nucleophile, and the cyano group can participate in the Michael addition reaction, and the conjugation addition with α,β-unsaturated carbonyl compounds can be used to construct new carbon-carbon bonds, which provides an effective way for the synthesis of complex organic molecules. Ethyl ester groups can be hydrolyzed under acidic or alkaline conditions to form corresponding carboxylic acids, which are key in the conversion of functional groups in organic synthesis.
In terms of preparation method, ethyl chloroacetate and sodium cyanide are commonly used as raw materials to prepare through nucleophilic substitution reaction, but this process needs to strictly control the dosage and reaction conditions of sodium cyanide, because of its high toxicity and improper operation, it is easy to cause safety accidents, and it is also necessary to pay attention to the follow-up purification steps to obtain high-purity products.
In industrial applications, it is a key intermediate in the synthesis of fine chemicals such as pharmaceuticals, pesticides, and fragrances. In medicine, it is used to manufacture sedative-hypnotic drugs such as barbiturates; In the field of pesticides, participate in the synthesis of compounds with insecticidal and herbicidal activities; In the synthesis of fragrances, it can build the skeleton of special flavor molecules and provide unique raw materials for the blending of various flavors, which plays a pivotal role in supporting the modern industry, agriculture and consumer goods industries.
It should be emphasized that due to the cyano group, Ethyl cyanoacetate has a certain toxicity and irritating effect on the skin, eyes, respiratory tract, etc., so it is necessary to wear protective equipment in a well-ventilated environment during operation, and strictly follow the safety regulations of chemical laboratories and chemical production.