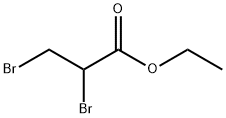
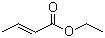Ethyl crotonate(CAS#623-70-1)
| Risk Codes | R11 – Highly Flammable R34 – Causes burns R36 – Irritating to the eyes |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S9 – Keep container in a well-ventilated place. S33 – Take precautionary measures against static discharges. |
| UN IDs | UN 1862 3/PG 2 |
| WGK Germany | 2 |
| RTECS | GQ3500000 |
| TSCA | Yes |
| HS Code | 29161980 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 3000 mg/kg |
Introduction
Ethyl trans-butenoate is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of the compound:
Quality:
Ethyl trans-butenoate is a colorless liquid with a peculiar odor. It is slightly denser than water with a density of 0.9 g/mL. Soluble in a variety of organic solvents, such as ethanol, ethers and naphthenes, at room temperature.
Use:
Ethyl trans-butenate has a variety of applications in the chemical industry. The most common use is as an intermediate in organic synthesis for the preparation of other organic compounds, such as oxalates, ester solvents and polymers. It can also be used as coatings, rubber adjuvants, and solvents.
Method:
The preparation method of trans-butenoate ethyl ester is generally obtained by the reaction of trans-butenoic acid with ethanol. This product is obtained by heating trans-butenic acid and ethanol under acidic conditions to form an ester.
Safety Information:
Ethyl trans-butenoate is irritating to the eyes and skin and may cause inflammation of the eyes and skin. Inhalation of its vapours should be avoided when handling the compound, and operations should be carried out in a well-ventilated area. When storing, it should be placed in an airtight container, away from ignition and oxidizers.