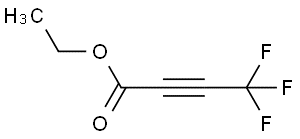
ETHYL 4 4 4-TRIFLUORO-2-BUTYNOATE(CAS# 79424-03-6)
| Risk Codes | R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 3272 3/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 19 |
| HS Code | 29161900 |
| Hazard Note | Irritant/Highly Flammable |
| Hazard Class | 3.1 |
| Packing Group | II |
Introduction
ETHYL 4,4,4-TRIFLUORO-2-BUTYNOATE(ETHYL 4,4,4-TRIFLUORO-2-BUTYNOATE) is an organic compound. The following is a description of its nature, use, preparation and safety information:
Nature:
-Appearance: It is usually a colorless liquid or yellowish liquid.
-Solubility: It can be dissolved in organic solvents, such as ethanol, ether and dichloromethane.
-Melting point and boiling point: Its melting point is about -8°C, and its boiling point is about 108-110°C.
Use:
-reagent in Advanced Organic Synthesis: ETHYL 4,4, 4-trifluororo-2-butynoate can be used as an important reagent in organic synthesis. It can participate in a variety of organic reactions, such as acylation, condensation and cyclization reactions, used to synthesize a variety of organic compounds.
-Material chemistry: It can also be used for certain reactions in polymer chemistry, such as crosslinking agents for synthetic polymers.
Method:
ETHYL 4,4,4-TRIFLUORO-2-BUTYNOATE can be prepared by the following steps:
1. First, butynol (2-butynol) is reacted with anhydrous hydrogen fluoride to generate butynyl fluoride.
2. Then, the butynyl fluoride is reacted with ETHYL chloroacetate to generate ETHYL 4,4, 4-trifluororo-2-butynoate.
Safety Information:
- ETHYL 4,4,4-TRIFLUORO-2-BUTYNOATE should avoid prolonged exposure to the air because it is sensitive to moisture and water.
-It should avoid open flames and high temperatures during operation and storage, because it is flammable.
-Appropriate protective measures should be taken when using and handling it, including wearing gloves, masks and protective glasses.
-It should be stored in a cool, dry and well-ventilated place.