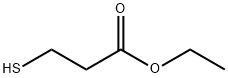
Ethyl 3-Mercaptopropionate(CAS#5466-6-8)
Ethyl 3-Mercaptopropionate(CAS#5466-6-8) Introduction
Physical:
Appearance: Usually colorless to light yellow transparent liquid with a special odor.
Boiling Point: Generally at 190 – 192 °C (at standard atmospheric pressure), the boiling point range may vary slightly depending on the experimental conditions and purity.
Density: The relative density is about 1.07 (water = 1), which means it is slightly heavier than water and during storage and use, it will be in the lower layer if mixed with water.
Solubility: slightly soluble in water, but miscible with most organic solvents such as ethanol, ether, acetone, etc., which makes it widely involved in the reaction of various solvent systems in organic synthesis reactions.
Chemical properties:
Functional group reactivity: The sulfhydryl group (-SH) in the molecule has strong reactivity and is the active site of many chemical reactions. It can undergo condensation reaction with carbonyl compounds such as aldehydes and ketones to form thioether compounds; It can also undergo substitution reactions with halogenated hydrocarbons to form new carbon-sulfur bonds, which can be used to construct complex organic molecular structures.
Stability: It is relatively stable at room temperature and pressure, but under the conditions of light, high temperature or the presence of strong oxidants, the sulfhydryl groups may be oxidized, resulting in changes in the chemical properties of the compounds, so they need to be stored and used under suitable conditions, and it is generally recommended to store in a cool, ventilated and dark environment, and avoid contact with strong oxidants.
Synthesis Method:
It is usually prepared by the esterification of 3-mercaptopropionic acid with ethanol in the presence of an acidic catalyst such as concentrated sulfuric acid. During the reaction, first of all, the carboxyl group and the hydroxyl group of ethanol undergo a nucleophilic substitution reaction under acidic conditions to form ester bonds, and at the same time water is generated. At the end of the reaction, the product needs to be purified through a series of post-processing steps such as neutralization, water washing, and distillation to obtain high-purity Ethyl 3-Mercaptopropionate.
Use:
Fragrance field: It has a unique odor and is used as an intermediate in synthetic fragrances in the fragrance industry, which can add special flavor and layering to the blended flavors, and is often used to blend flavors such as fruits and meats to meet the needs of food, cosmetics and other industries for fragrance diversification.
Pharmaceutical field: It can be used as a raw material or intermediate in drug synthesis to construct molecular structures with specific biological activities. For example, in the synthesis of some sulfur-containing drugs, their sulfhydryl groups can be introduced into the target molecule through chemical reactions, thereby imparting specific pharmacological activities to the drug, such as antioxidant, antimicrobial, or regulating enzyme activity.
Agriculture: It also has a certain application in the synthesis of pesticides, by modifying its molecular structure and introducing specific active groups, so that it can show good control effects on pests or pathogens on crops, which helps to improve the yield and quality of crops and ensure the stability of agricultural production.