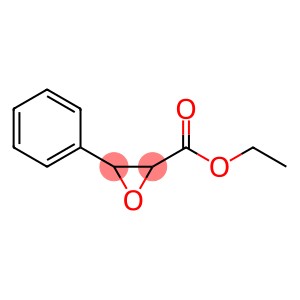
Ethyl 2,3-epoxy-3-phenylpropionate(CAS#121-39-1)
Risk and Safety
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S23 – Do not breathe vapour. S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 2 |
| RTECS | MB4970000 |
| TSCA | Yes |
| HS Code | 29189990 |
| Toxicity | The acute oral LD50 value in rats was reported as 2.3 ml/kg (2.0-2.6 ml/ kg) (Shelanski, 1973b). The acute dermal LD50 value in rabbits was reported as > 5 g/kg (Shelanski, 1973a). |
Ethyl 2,3-epoxy-3-phenylpropionate(CAS#121-39-1) introduction
Ethyl 2,3-epoxy-3-phenylpropionate, also known as ethyl phenylepoxydicarboxylate, is an organic compound. The following is information about its properties, uses, manufacturing methods and safety:
Quality:
Appearance: Colorless liquid.
Density: 1.107 g/cm³.
Solubility: Soluble in organic solvents such as ethanol and ether.
Use:
Ethyl 2,3-epoxy-3-phenylpropionate has important uses in the field of chemistry and is mainly used in the following areas:
As a catalyst and intermediate in organic synthesis to synthesize other organic compounds.
It can be used as a photoinitiator and an additive in coatings.
Method:
The preparation method of ethyl 2,3-epoxy-3-phenylpropionate mainly comprises the following steps:
Benzoic acid and ethyl chloroacetate were added to the reaction tungsten sand catalyst to obtain ethyl phenylacetate by esterification.
Then, ethyl phenylacetate is reacted with hydrogen peroxide under temperature control to generate ethyl 2,3-epoxy-3-phenylpropionate.
Safety Information:
Ethyl 2,3-epoxy-3-phenylpropionate is a chemical that should be handled with protective glasses, gloves and protective clothing to avoid contact with the skin.
Avoid inhaling gases or vapours and operate in a well-ventilated area.
Avoid contact with oxidants and strong acids to avoid dangerous reactions.
If inhaled or ingested, seek medical attention immediately.