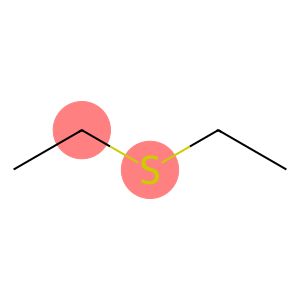
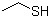Ethanethiol(CAS#75-08-1)
| Risk Codes | R11 – Highly Flammable R20 – Harmful by inhalation R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R12 – Extremely Flammable R20/22 – Harmful by inhalation and if swallowed. |
| Safety Description | S16 – Keep away from sources of ignition. S25 – Avoid contact with eyes. S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 2363 3/PG 1 |
| WGK Germany | 3 |
| RTECS | KI9625000 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 2930 90 98 |
| Hazard Class | 3 |
| Packing Group | I |
| Toxicity | LD50 orally in Rabbit: 682 mg/kg |
Introduction
Ethyl mercaptan. The following is an introduction to the properties, uses, preparation methods and safety information of ethyl mercaptan:Quality:- Appearance: Colorless liquid with a foul-smelling taste.- Density: approx. 0.840 g/cm³.- Solubility: Soluble in water, alcohols, and certain organic solvents.Use:- Chemical synthesis: Ethyl mercaptan can be used as an intermediate in the synthesis of organic compounds, and is often used as a reducing agent, sulfiding agent or stabilizer in organic synthesis reactions.Method:- Industrial production: Ethyl mercaptans are generally obtained by the reaction of sodium hydrosulfide and acetonylated hydrocarbons. The specific reaction conditions and steps are determined according to the actual situation of the reaction system.- Laboratory preparation: Ethyl mercaptan can be obtained by the reaction of ethyl magnesium bromide and hydrogen sulfide.Safety Information:- Ethyl mercaptan has a strong odor and strong irritation, so care should be taken to avoid inhaling its vapors or touching the skin.- Toxic sulfide gases are released when burned, so avoid contact with ignition sources.- Ethyl mercaptan is irritating to the eyes and skin, and direct contact should be avoided during use.- Avoid contact with oxidants and strong acids during storage to prevent dangerous reactions.