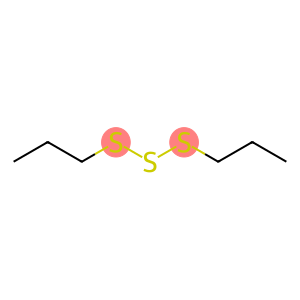
Dipropyl trisulfide(CAS#6028-61-1)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | 22 – Harmful if swallowed |
| WGK Germany | 3 |
| RTECS | UK3870000 |
Introduction
Dipropyltrisulfide is an organic compound. The following is an introduction to its nature, use, preparation method and safety information:
Quality:
- Dipropyl trisulfide is a colorless liquid with a special sulfur taste.
- It is insoluble in water but can be soluble in organic solvents such as ethers, ethanol and ketone solvents.
Use:
- Dipropyltrisulfide is commonly used as a vulcanizing agent in organic synthesis to introduce sulfur atoms into organic molecules.
- It can be used to synthesize sulfur-containing organic compounds such as thioketones, thioates, etc.
- It can also be used as a rubber processing aid to improve the heat resistance and aging resistance of rubber.
Method:
- Dipropyl trisulfide is usually prepared by a synthetic reaction. A commonly used preparation method is to react dipropyl disulfide with sodium sulfide under alkaline conditions.
- The reaction equation is: 2(CH3CH2)2S + Na2S → 2(CH3CH2)2S2Na → (CH3CH2)2S3.
Safety Information:
- Dipropyl trisulfide has a pungent odor and may be irritating to the eyes, skin, and respiratory system upon contact.
- Wear appropriate personal protective equipment such as protective gloves, goggles, and protective masks when using.
- Avoid contact with ignition sources and avoid sparks or electrostatic discharges to prevent fire or explosion.
- Use in a well-ventilated area to avoid inhaling vapors. In case of inhalation or exposure, seek medical attention immediately and provide information about the chemical.