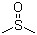Dimethyl sulfoxide(CAS#67-68-5)
Risk and Safety
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S24/25 – Avoid contact with skin and eyes. S37/39 – Wear suitable gloves and eye/face protection S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S23 – Do not breathe vapour. |
| UN IDs | NA 1993 / PGIII |
| WGK Germany | 1 |
| RTECS | PV6210000 |
| FLUKA BRAND F CODES | 3 |
| TSCA | Yes |
| HS Code | 29309070 |
| Toxicity | LD50 orally in rats: 17.9 ml/kg (Bartsch) |
Dimethyl sulfoxide(CAS#67-68-5)
quality
It is a colorless and transparent liquid at room temperature. Strong hygroscopicity. Soluble in water, ethanol, acetone, ether and chloroform, it is a highly polar inert solvent. It is flammable in case of open flame and high heat. Thermal decomposition produces toxic sulfide fumes. It can have a violent chemical reaction with halides such as acid chloride, trichlorosilane, phosphorus trichloride, etc.
Method
Methanol and carbon disulfide are used as raw materials to prepare dimethyl sulfide, and dimethyl sulfoxide is obtained by oxidation and refining of dimethyl sulfide.
use
Methyl sulfoxide is an extremely important aprotic polar solvent that is soluble in both water and organic solvents. It is widely used as a solvent and reaction reagent, and has a high selective extraction ability. It can be used as an extraction solvent for the separation of alkanes and aromatic hydrocarbons; used as a synthetic solvent for polyamide, fluchloroaniline, polyimide and polysulfone resins; It is also used as a solvent for acetylene, aromatics, sulfur dioxide and other gases. DMSO has anti-inflammatory, analgesic, diuretic, sedative and other effects, and dimethyl sulfoxide can also be directly used as a raw material and carrier for some drugs in the pharmaceutical industry, which has strong permeability to the skin and helps the drug to penetrate into the human body. It can also be used as capacitive medium, antifreeze, brake fluid, rare metal extractant, etc.
security
rat oral LDso:17. 9mL/kg。 It is harmful to the body when inhaled, ingested, or absorbed through the skin. It has an irritating effect on the eyes, skin, mucous membranes and upper respiratory tract. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. It should be stored separately from oxidants, reducing agents, halides, and acids, and should not be mixed.