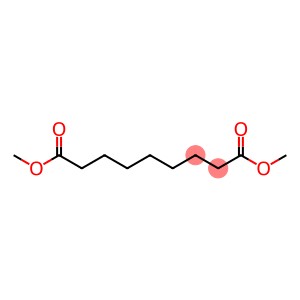
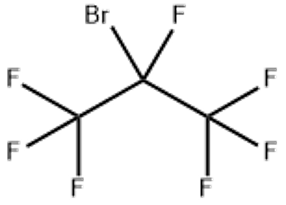
Dimethyl azelate(CAS#1732-10-1)
| Safety Description | S23 – Do not breathe vapour. S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 1 |
| TSCA | Yes |
| HS Code | 29171310 |
Introduction
Dimethyl azelaic acid (also known as Dioctyl adipate, DOA) is a common organic compound. The following is an introduction to its nature, use, manufacturing methods and safety information:
Quality:
- Appearance: Colorless to yellowish liquid
- Solubility: soluble in organic solvents, slightly soluble in water
- Refractive index: approx. 1.443-1.449
Use:
- Dimethyl azelarate is mainly used as a plasticizer, which has good plasticity and cold resistance, and can increase the softness and cold resistance of plastics.
- It is often used in the production of polyvinyl chloride (PVC) plastics, synthetic rubber, synthetic resins, etc., to improve their plasticity and strength.
- Dimethyl azelaate can also be used as a lubricant, softener and antifreeze, among other things.
Method:
Dimethyl azelaic acid is usually prepared by esterification reaction as follows:
1. React nonanediol with adipic acid.
2. Add esterifying agents, such as sulfuric acid, as catalysts in the esterification reaction.
3. The reaction is carried out under the appropriate temperature and pressure conditions to generate dimethyl azelaate.
4. The product is further purified by dehydration, distillation and other steps.
Safety Information:
- Dimethyl azelaic acid should be protected under normal use conditions and avoid contact with skin and eyes.
- Wear appropriate protective equipment, including respiratory protection and protective gloves, if used.
- Attention should be paid to a well-ventilated environment during operation to avoid inhalation or accidental ingestion.
- During storage and transportation, it is necessary to prevent contact with oxidants, acids and other substances to avoid dangerous accidents.