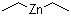Diethylzinc(CAS#557-20-0)
| Risk Codes | R14 – Reacts violently with water R17 – Spontaneously flammable in air R34 – Causes burns R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R67 – Vapors may cause drowsiness and dizziness R65 – Harmful: May cause lung damage if swallowed R62 – Possible risk of impaired fertility R48/20 - R11 – Highly Flammable R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R14/15 - R63 – Possible risk of harm to the unborn child |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label. S8 – Keep container dry. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S16 – Keep away from sources of ignition. S60 – This material and its container must be disposed of as hazardous waste. S43 – In case of fire use … (there follows the type of fire-fighting equipment to be used.) |
| UN IDs | UN 3399 4.3/PG 1 |
| WGK Germany | 2 |
| RTECS | ZH2077777 |
| FLUKA BRAND F CODES | 10-23 |
| TSCA | Yes |
| HS Code | 29319090 |
| Hazard Class | 4.3 |
| Packing Group | I |
Introduction
Diethyl zinc is an organozinc compound. It is a colorless liquid, flammable and has a pungent odor. The following is an introduction to the properties, uses, preparation methods and safety information of diethylzinc:
Quality:
Appearance: Colorless liquid with pungent odor
Density: approx. 1.184 g/cm³
Solubility: soluble in organic solvents such as ethanol and benzene
Use:
Diethyl zinc is an important reagent in organic synthesis and is used in the preparation of catalysts.
It can also be used as an inducer and reducing agent for olefins.
Method:
By reacting zinc powder with ethyl chloride, diethyl zinc is generated.
The preparation process needs to be carried out under the protection of an inert gas (e.g. nitrogen) and at low temperatures to ensure the safety and high yield of the reaction.
Safety Information:
Diethyl zinc is highly flammable and contact with an ignition source may cause a fire or explosion. Fire and explosion prevention measures must be taken during storage and use.
Wear appropriate protective equipment such as chemical protective clothing, protective glasses, and gloves when using.
Avoid contact with strong oxidants and acids to prevent violent reactions.
Diethylzinc should be handled in a well-ventilated area to reduce the accumulation of harmful gases.
Store tightly sealed and place in a dry, cool place to prevent unstable conditions.