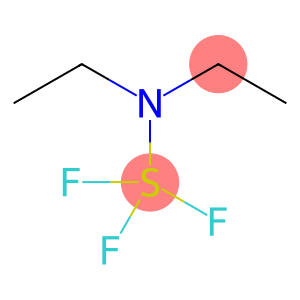
Diethylaminosulfur trifluoride(CAS#38078-09-0)
| Risk Codes | R10 – Flammable R14 – Reacts violently with water R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R34 – Causes burns R5 – Heating may cause an explosion R35 – Causes severe burns R40 – Limited evidence of a carcinogenic effect |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 2920 8/PG 1 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 3-10-21 |
| HS Code | 29309070 |
| Hazard Note | Corrosive/Keep Cold |
| Hazard Class | 8 |
| Packing Group | I |
Introduction
Diethylaminosulfur trifluoride (also known as BET) is an organic compound. The following is an introduction to some of its properties, uses, manufacturing methods and safety information:Quality:- BET is a colorless liquid with a pungent odor.- It is soluble in organic solvents such as methanol, acetone, and chloroform, and insoluble in water.- BET is stable and can be stored at room temperature.Use:- BET is commonly used in organic synthesis reactions as a fluorosulfur reagent.- It can be used to introduce trifluoromethanyl groups (CF3) into organic molecules, thereby altering their properties and reactivity.Method:- A common method for preparing BET is to react diethylaminosulfur ((C2H5)2SNH) with fluorine gas at the appropriate temperature and pressure to obtain the target product.- Reactions to prepare BET usually need to be carried out in an inert atmosphere (e.g., nitrogen) to prevent adverse reactions of reactants or products with oxygen or moisture in the air.Safety Information:- When using BET, it is necessary to operate in a well-ventilated laboratory or under an exhaust hood to prevent the accumulation of harmful gases.- Exposure to BET may cause eye and skin irritation, so personal protective equipment such as protective gloves, goggles, and lab coats should be worn.- When handling and disposing of waste, follow relevant laws and regulations to avoid potential harm to the environment and human health.


![2-[(1R)-3-(dipropan-2-ylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol(CAS#207679-81-0)](https://www.xinchem.com/uploads/phenol.gif)
![(3S,7aR)-3-(trichloroMethyl)tetrahydropyrrolo[1,2-c]oxazol-1(3H)-one(CAS#1330286-50-4)](https://www.xinchem.com/uploads/3-trichloroMethyltetrahydropyrrolo12-coxazol-13H-one.gif)