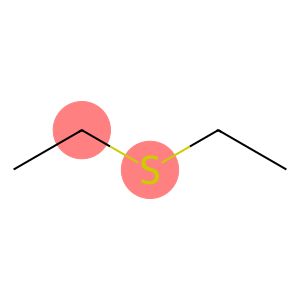
Diethyl sulfide(CAS#352-93-2)
| Risk Codes | R11 – Highly Flammable R38 – Irritating to the skin R36/38 – Irritating to eyes and skin. |
| Safety Description | S9 – Keep container in a well-ventilated place. S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S33 – Take precautionary measures against static discharges. S36 – Wear suitable protective clothing. |
| UN IDs | UN 2375 3/PG 2 |
| WGK Germany | 3 |
| RTECS | LC7200000 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 29309090 |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
Ethyl sulfide is an organic compound. The following is an introduction to the properties, uses, manufacturing methods and safety information of ethyl sulfide:
Quality:
- Appearance: Ethyl sulfide is a colorless liquid with an unpleasant odor.
- Solubility: It is soluble in organic solvents such as alcohols and ethers, but insoluble in water.
- Thermal stability: Ethyl sulfide can decompose at higher temperatures.
Use:
- Ethyl sulfide is mainly used as a solvent in organic synthesis. It can be used as an ether-based reagent or sulfur shaker reagent in many reactions.
- It can also be used as a solvent for certain polymers and pigments.
- High-purity ethyl sulfide can be used for catalytic reduction reactions in organic synthesis.
Method:
- Ethyl sulfide can be obtained by the reaction of ethanol with sulfur. This reaction is usually carried out under alkaline conditions, such as with alkali metal salts or alkali metal alcohols.
- A common method for this reaction is to react ethanol with sulfur through a reducing agent such as zinc or aluminum.
Safety Information:
- Ethyl sulfide is a flammable liquid with a low flash point and autoignition temperature. Care should be taken to avoid contact with flames, high temperatures, or sparks. In case of accidental contact, rinse immediately with soap and water.
- When handling ethyl sulfide, it is essential to maintain a well-ventilated laboratory environment to avoid the risk of explosion or poisoning due to the accumulation of vapors.
- Ethyl sulfide is irritating to the eyes and respiratory system, and appropriate protective equipment such as gloves, goggles, and protective masks should be worn when operating.