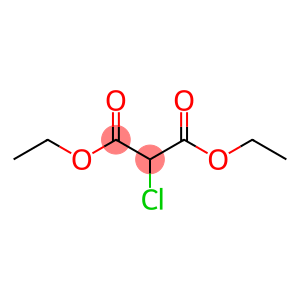
diethyl chloromalonate(CAS#14064-10-9)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R36/37 – Irritating to eyes and respiratory system. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S27 – Take off immediately all contaminated clothing. S28 – After contact with skin, wash immediately with plenty of soap-suds. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| HS Code | 29171990 |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
Diethyl chloromalonate (also known as DPC). The following is an introduction to the properties, uses, preparation methods and safety information of diethyl chloromalonate:
1. Nature:
- Appearance: Diethyl chloromalonate is a colorless liquid.
- Solubility: It is soluble in most organic solvents, such as alcohols, ethers, and aromatic hydrocarbons, but slightly soluble in water.
- Stability: It is relatively stable to light and heat, but can produce toxic hydrogen chloride gas at high temperatures or open flames.
2. Usage:
- As a solvent: Diethyl chloromalonate can be used as a solvent, especially in organic synthesis to dissolve and react organic compounds.
- Chemical synthesis: It is a commonly used reagent for the synthesis of esters, amides, and other organic compounds.
3. Method:
- Diethyl chloromalonate can be obtained by the reaction of diethyl malonate with hydrogen chloride. The reaction conditions are generally at room temperature, hydrogen chloride gas is introduced into diethyl malonate, and a catalyst is added to promote the reaction.
- Reaction equation: CH3CH2COOCH2CH3 + HCl → ClCH2COOCH2CH3 + H2O
4. Safety Information:
- Diethyl chloromalonate has a pungent odor and may cause irritation to the skin, eyes, and respiratory tract.
- It is a flammable liquid that needs to be stored in a cool, well-ventilated place and away from fire sources and open flames.
- Appropriate personal protective equipment such as gloves, goggles, and protective clothing should be worn during handling.