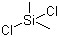Dichlorodimethylsilane(CAS#75-78-5)
| Risk Codes | R20 – Harmful by inhalation R59 – Dangerous for the ozone layer R36/37/38 – Irritating to eyes, respiratory system and skin. R11 – Highly Flammable R67 – Vapors may cause drowsiness and dizziness R65 – Harmful: May cause lung damage if swallowed R63 – Possible risk of harm to the unborn child R48/20 - R38 – Irritating to the skin R20/21 – Harmful by inhalation and in contact with skin. R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R37 – Irritating to the respiratory system R35 – Causes severe burns R20/22 – Harmful by inhalation and if swallowed. R14 – Reacts violently with water R34 – Causes burns |
| Safety Description | S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label. S36/37 – Wear suitable protective clothing and gloves. S59 – Refer to manufacturer / supplier for information on recovery / recycling. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S16 – Keep away from sources of ignition. S7/9 - S2 – Keep out of the reach of children. |
| UN IDs | UN 2924 3/PG 2 |
| WGK Germany | 3 |
| RTECS | VV3150000 |
| FLUKA BRAND F CODES | 3-10-19-21 |
| TSCA | Yes |
| HS Code | 29310095 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 6056 mg/kg |
Introduction
Dimethyldichlorosilane is an organosilicon compound.
Quality:
1. Appearance: colorless or light yellow liquid.
2. Solubility: soluble in organic solvents, such as alcohols and esters.
3. Stability: It is stable at room temperature, but can decompose when heated.
4. Reactivity: It can react with water to form silica alcohol and hydrochloric acid. It can also be replaced with ethers and amines.
Use:
1. As an initiator: In organic synthesis, dimethyldichlorosilane can be used as an initiator to initiate certain polymerization reactions, such as the synthesis of silicon-based polymers.
2. As a cross-linking agent: Dimethyl dichlorosilane can react with other compounds to form a cross-linked structure, which is used to prepare elastomer materials such as silicone rubber.
3. As a curing agent: In coatings and adhesives, dimethyldichlorosilane can react with polymers containing active hydrogen to cure and increase the weather resistance of materials.
4. Used in organic synthesis reactions: Dimethyldichlorosilane can be used to synthesize other organosilicon compounds in organic synthesis.
Method:
1. It is obtained from the reaction of dichloromethane and dimethylchlorosilanol.
2. It is obtained from the reaction of methyl chloride silane and methyl magnesium chloride.
Safety Information:
1. It is irritating and corrosive, rinse immediately with plenty of water and seek medical assistance when it comes into contact with skin and eyes.
2. Avoid inhaling its vapors when using it to ensure good ventilation.
3. Keep away from fire sources and oxidants, keep the container airtight, and store in a cool, dry place.
4. Do not mix with acids, alcohols and ammonia to avoid dangerous reactions.
5. When disposing of waste, comply with relevant regulations and safety operation guidelines.