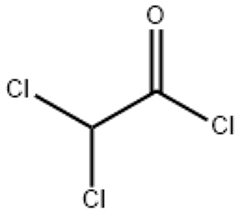
Dichloracetylchlorid(CAS# 79-36-7)
| Risk Codes | R35 – Causes severe burns R50 – Very Toxic to aquatic organisms |
| Safety Description | S9 – Keep container in a well-ventilated place. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 1765 8/PG 2 |
| WGK Germany | 2 |
| RTECS | AO6650000 |
| FLUKA BRAND F CODES | 19-21 |
| TSCA | Yes |
| HS Code | 29159000 |
| Hazard Note | Corrosive/Moisture Sensitive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Dichloroacetyl chloride is an organic compound. The following is an introduction to its properties, uses, manufacturing methods and safety information:
Quality:
Appearance: Dichloroacetyl chloride is a colorless liquid.
Density: The density is relatively high, about 1.35 g/mL.
Solubility: Dichloroacetyl chloride can be dissolved in most organic solvents, such as ethanol, ether and benzene.
Use:
Dichloroacetyl chloride can be used as a chemical reagent and is often used in organic synthesis.
Similarly, dichloroacetyl chloride is one of the important raw materials for the synthesis of pesticides.
Method:
The general method of preparing dichloroacetyl chloride is the reaction of dichloroacetic acid and thionyl chloride. Under reaction conditions, the hydroxyl group (-OH) in dichloroacetic acid will be replaced by chlorine (Cl) in thionyl chloride to form dichloroacetyl chloride.
Safety Information:
Dichloroacetyl chloride is an irritating substance and should be avoided from direct contact with the skin and eyes.
When using dichloroacetyl chloride, gloves, protective eyewear, and protective clothing should be worn to avoid unnecessary risks.
It should be used in a well-ventilated area to prevent inhalation of gases.
Waste should be disposed of properly in accordance with local regulations.