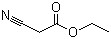
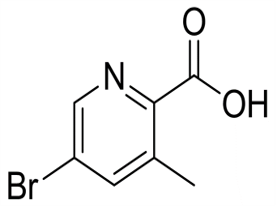
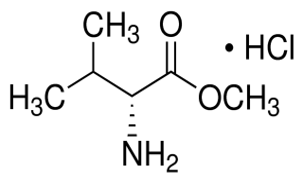
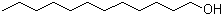Dibromomethane(CAS#74-95-3)
| Risk Codes | R20 – Harmful by inhalation R52/53 – Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R11 – Highly Flammable |
| Safety Description | S24 – Avoid contact with skin. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S16 – Keep away from sources of ignition. S7 – Keep container tightly closed. |
| UN IDs | UN 2664 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | PA7350000 |
| TSCA | Yes |
| HS Code | 2903 39 15 |
| Hazard Class | 6.1 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 108 mg/kg LD50 dermal Rabbit > 4000 mg/kg |
Introduction
Dibromomethane. The following is an introduction to the properties, uses, preparation methods and safety information of dibromomethane:
Quality:
It has a pungent odor at room temperature and is insoluble in water, but soluble in many common organic solvents.
Dibromomethyl is a chemically stable substance that does not decompose or undergo chemical reactions easily.
Use:
Dibromomethane is often used as a solvent for organic synthesis reactions, dissolving or extracting lipids, resins and other organic substances.
Dibromomethane is also used as a raw material for the preparation of other organic compounds, and has applications in some industrial processes.
Method:
Dibromomethane is usually prepared by reacting methane with bromine.
Under the reaction conditions, bromine is able to replace one or more hydrogen atoms in methane to form dibromomethane.
Safety Information:
Dibromomethane is toxic and can be absorbed by inhalation, skin contact, or ingestion. Long-term exposure may have adverse health effects.
Appropriate personal protective equipment such as gloves, goggles, and face shields should be worn when in use.
Care should be taken to avoid contact with ignition sources when handling and storing dibromomethane, as it is flammable.
Dibromomethane should be stored away from heat sources and high temperatures in a cool, well-ventilated place.
When using, storing or handling dibromomethane, safe operating procedures should be strictly followed to ensure personal safety. In case of accidents, appropriate emergency measures should be taken.