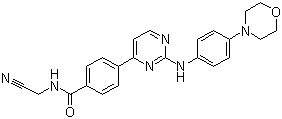
CYT 387(CAS#1056634-68-4)
CYT 387(CAS#1056634-68-4) Introduction
Momelotinib (CYT387) is an ATP competitive JAK1/JAK2 inhibitor with IC50 of 11 nM/18 nM, which is about 10 times more selective than JAK3.
pharmachologic effect
It is an ATP-competitive JAK1/JAK2 inhibitor with IC50s of 11 nM and 18 nM for JAK1 and JAK2, respectively, and selectivity for JAK3 is about 1/10 of that of JAK1 and JAK2, and can effectively inhibit the JAK-STAT signaling pathway, which is closely related to various biological processes such as cell proliferation, differentiation, and apoptosis, and can play a role in regulating immunity, anti-inflammatory, and anti-proliferation by inhibiting this pathway.345
Clinical application
It was approved by the FDA in September 2023 for the treatment of adult anemia with intermediate- or high-risk myelofibrosis (MF), including primary myelofibrosis or secondary myelofibrosis, i.e., polycythemia vera and essential thrombocythemia.
Adverse effects
The most common adverse reactions were thrombocytopenia, bleeding, bacterial infection, fatigue, dizziness, diarrhea and nausea.
Storage condition 5
Powder form can be stored at -20°C for 3 years and 4°C for 2 years.
After dissolving in solvents, it can be stored at -80°C for 6 months, and -20°C is recommended for 1 month.
use
It is used in cell experiments and animal experiments in scientific research to study JAK1/JAK2-related signaling pathways and disease mechanisms. Preclinical studies have shown potential for the treatment of diseases such as myelofibrosis, improving associated symptoms and improving patients’ quality of life134.
Synthesis method
It is usually prepared from 4-(4-morpholino)aniline and 4-chloropyrimidine as starting materials, and is prepared by multi-step reactions, such as condensation, substitution, and amidation.
CYT 387 is an important JAK inhibitor and has important implications in the treatment of myelofibrosis and other diseases, but its specific use should be carried out under the guidance of a physician.


![5-tert-butyl 2-ethyl 6,7-dihydrothiazolo[5,4-c]pyridine-2,5(4H)-dicarboxylate(CAS#1053656-51-1)](https://www.xinchem.com/uploads/dihydrothiazolopyridinedicarboxylate.gif)