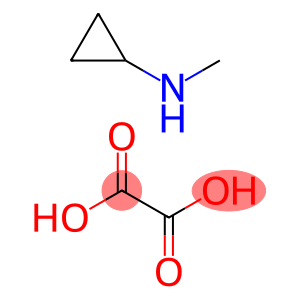
Cyclopropanamine, N-methyl-, ethanedioate (1:1)(CAS#24571-78-6)
Cyclopropanamine, N-methyl-, ethanedioate (1:1)(CAS#24571-78-6)
Physicochemical properties
Appearance: Usually white crystalline powder.
Solubility: It can be dissolved in water to a certain extent, and its solubility in organic solvents may be limited. Oxalate usually has some water solubility due to interactions such as hydrogen bonds that can form between oxalate ions and water molecules.
Structural features
Salts formed by the acid-base neutralization reaction of N-methylcyclopropylamine and oxalic acid in a ratio of 1:1. The N-methylcyclopropylamine moiety contains cyclopropyl and methyl groups, which have a certain rigid structure and steric hindrance.
The oxalate ion (C₂O₄²⁻) acts as an anionic moiety and binds to the ammonium ion (-NH₃⁺) of N-methylcyclopropylamine by ionic bonding.
Fields of application
Pharmaceutical field: It can be used as a pharmaceutical intermediate for the synthesis of some pharmaceutical molecules with special structure and biological activity. For example, in the development of some new antimicrobial drugs or neuromodulatory drugs, the structural characteristics of N-methylcyclopropylamine oxalate may be used to introduce specific groups or structural fragments to improve the activity, selectivity and pharmacokinetic properties of the drug.
Organic synthesis: It is an important intermediate in organic synthesis, which can participate in a variety of organic reactions and is used to construct more complex organic compound structures. For example, organic compounds with different functions and structures can be prepared by reacting with other compounds containing specific functional groups, such as substitution and addition, which provides important basic raw materials for the research and development of organic synthetic chemistry.
Safety Information
As a chemical substance, it needs to be safe when storing and using it. Contact with strong oxidants, strong acids, strong alkalis and other substances should be avoided to avoid chemical reactions.
During use, appropriate protective equipment such as gloves, goggles, etc., should be worn to avoid skin contact and eye contact.