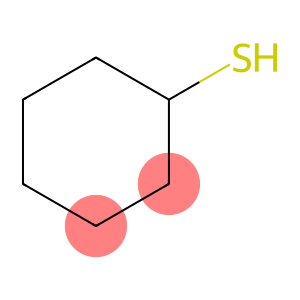
Cyclohexyl mercaptan(CAS#1569-69-3)
| Risk Codes | R10 – Flammable R20/22 – Harmful by inhalation and if swallowed. R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S57 – Use appropriate container to avoid environmental contamination. S37/39 – Wear suitable gloves and eye/face protection S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S16 – Keep away from sources of ignition. |
| UN IDs | UN 3054 3/PG 3 |
| WGK Germany | 3 |
| RTECS | GV7525000 |
| HS Code | 29309070 |
| Hazard Note | Irritant/Flammable/Stench/Air Sensitive |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Cyclohexanethiol is an organosulfur compound. The following is an introduction to the properties, uses, preparation methods and safety information of cyclohexanol:
Quality:
Appearance: Colorless liquid with a strong foul-smelling odor.
Density: 0.958 g/mL.
Surface tension: 25.9 mN/m.
It gradually turns yellow when exposed to sunlight.
Soluble in most organic solvents.
Use:
Cyclohexanol is widely used in chemical synthesis as a desulfurization reagent and a precursor for sulfur-containing compounds.
In organic synthesis, it can be used as a catalyst and reaction intermediate.
Method:
Cyclohexanol can be prepared by the following reactions:
Cyclohexyl bromide reacts with sodium sulfide.
Cyclohexene reacts with sodium hydrosulfide.
Safety Information:
Cyclohexanol has a pungent odor that can cause sore throat and difficulty breathing.
Avoid direct contact with skin and eyes, and rinse with plenty of water if contact occurs.
Good ventilation should be maintained during use.
Cyclohexane has a low flash point and avoids contact with open flames and high temperatures.
It should be stored in an airtight container, away from fire and oxidants.