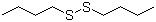Butyl disulfide(CAS#629-45-8)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S7/9 - S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | 2810 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 13 |
| HS Code | 29309070 |
| Hazard Class | 6.1(b) |
| Packing Group | III |
Introduction
N-butyl disulfide is an organosulfur compound. The following is an introduction to the properties, uses, preparation methods and safety information of n-butyl disulfide:- N-butyl disulfide is a colorless to light yellow liquid with a pungent odor. – It is soluble in organic solvents such as alcohols, ethers, and hydrocarbons, but almost insoluble in water. – N-butyl disulfide has a low ignition point, is flammable in the air, and can produce toxic sulfide gases. Uses:- N-butyl disulfide is mainly used as a vulcanization reagent in organic synthesis. – In chemical reactions, it can react with alkyl halides to form sulfides, which are used to synthesize compounds such as thioethers and thioesters. Preparation method:- N-butyl disulfide can be prepared by the reaction of n-butyl bromide and sodium sulfide in organic solvents. The reaction equation is as follows:2(CH3CH2CH2CH2Br) + Na2S → (CH3CH2CH2CH2)2S + 2NaBrSafety Information:- N-butyl disulfide is an irritant substance that can cause irritation and inflammation in contact with the skin and eyes. – It is volatile and needs to be used in a well-ventilated laboratory environment. – Personal protective equipment such as protective gloves, goggles, and lab coats are required during handling. – Avoid contact with fire sources and smoke inhalation. In case of inhalation, the victim should be moved immediately to a place with fresh air and medical attention should be sought as soon as possible. Please follow the safety operating procedures and refer to the detailed safety information provided by the supplier during use.