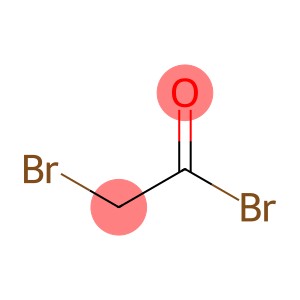
Bromoacetyl bromide(CAS#598-21-0)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R14 – Reacts violently with water |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S8 – Keep container dry. S30 – Never add water to this product. S25 – Avoid contact with eyes. |
| UN IDs | UN 2513 8/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 10-19 |
| TSCA | Yes |
| HS Code | 29159080 |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Bromoacetyl bromide is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of bromoacetyl bromide:
Quality:
Appearance: Bromoacetyl bromide is a colorless to pale yellow liquid.
Solubility: It is easily soluble in organic solvents, but it is difficult to dissolve in water.
Instability: Bromoacetyl bromide decomposes at high temperatures or humidity to produce toxic gases.
Use:
Bromoacetyl bromide is often used as a brominating reagent in organic synthesis, and it can be used as a brominating reagent for ketone-derived compounds.
It can also be used in the preparation of solvents, catalysts and surfactants.
Method:
Bromoacetyl bromide can be prepared by the reaction of bromoacetic acid with ammonium bromide in acetic acid:
CH3COOH + NH4Br + Br2 → BrCH2COBr + NH4Br + HBr
Safety Information:
Bromoacetyl bromide should be handled with protective measures, such as wearing protective glasses, gloves and lab coats.
It is a caustic compound that can cause irritation and burns in contact with the skin or eyes. Rinse with plenty of water immediately after exposure and seek medical attention.
When storing and using bromoacetyl bromide, it should be kept away from fire sources and open flames, and avoid high temperature environments to prevent explosions and the release of dangerous gases.