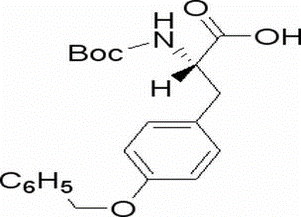
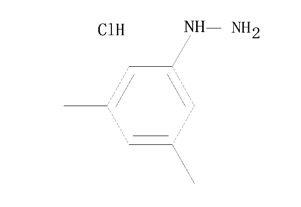
Boc-O-benzyl-L-tyrosine(CAS# 2130-96-3)
| Safety Description | S22 – Do not breathe dust. S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| HS Code | 29242990 |
Introduction
N-Boc-O-benzyl-L-tyrosine is an organic compound that contains N-Boc protecting group, benzyl group and L-tyrosine group in its chemical structure.
The following is about the properties of N-Boc-O-benzyl-L-tyrosine:
Physical properties: powdered solid, colorless or white.
Chemical Properties: The N-Boc protecting group is a protective group for the amino group, which can protect tyrosine in synthesis and reaction without being destroyed. Benzyl groups are aromatic groups with stable chemical properties. L-Tyrosine is an amino acid that has properties such as acidity, alkalinity, solubility, etc.
The main uses of N-Boc-O-benzyl-L-tyrosine include, but are not limited to:
The method of preparation of N-Boc-O-benzyl-L-tyrosine is usually by chemical synthesis. A common approach is to use L-tyrosine as the starting material and go through a series of reaction steps, including esterification and N-Boc protection, to finally obtain the target product.
When using N-Boc-O-benzyl-L-tyrosine, the following safety information should be noted:
Avoid contact with skin and eyes to avoid irritation or damage.
Avoid inhaling dust or solution vapors and operate in a well-ventilated environment.
Follow proper personal protective measures, such as wearing gloves, goggles, and protective clothing.
When storing, contact with oxidants or strong acids should be avoided to avoid dangerous reactions.
When using or handling, it is important to follow proper laboratory practices and follow relevant safety measures.