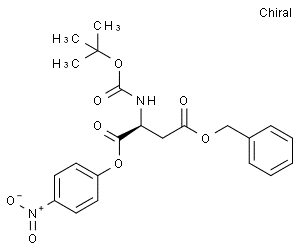
BOC-ASP(OBZL)-ONP(CAS# 26048-69-1)
BOC-ASP(OBZL)-ONP(CAS# 26048-69-1) Introduction
4-Benzyl1-(4-nitrophenyl)(tert-butoxycarbonyl)-L-aspartic acid is an organic compound. The following describes its properties, applications, manufacturing methods, and safety information.
Quality:
- Appearance: Usually white crystals or crystalline powder.
- Solubility: Soluble in some organic solvents such as methanol, methylene chloride and ethanol.
Use:
- It can be used as an amino acid protecting group for the synthesis of peptide sequences.
- Boc-L-Aspartic Acid 4-Benzyl 1-(4-Nitrophenyl)Ester can also be used to construct new bioactive molecules.
Method:
The preparation of 4-benzyl1-(4-nitrophenyl)(tert-butoxycarbonyl)-L-aspartic acid typically includes the following steps:
L-aspartic acid is esterified with Branstri chloride (Boc) to form Boc-L-aspartic acid.
Boc-L-aspartic acid is reacted with benzyl alcohol to produce 4-benzyl Boc-L-aspartic acid.
Under alkaline conditions, 4-benzyl Boc-L-aspartic acid is reacted with excess 4-nitrophenyl iodide to generate 4-benzyl1-(4-nitrophenyl)Boc-L-aspartic acid.
The target product, 4-benzyl1-(4-nitrophenyl)(tert-butoxycarbonyl)-L-aspartic acid, was obtained by deprotecting 4-benzyl1-(4-nitrophenyl)(tert-butoxycarbonyl)-L-aspartic acid by deprotecting (removing the Boc protecting group).
Safety Information:
- There is little safety data for this compound, but as an organic compound, care should be taken to prevent inhalation, skin contact, and ingestion.
- Appropriate personal protective equipment such as laboratory gloves, goggles, and protective masks should be worn during handling.
- It should be operated in a well-ventilated area to avoid the generation of dust.