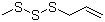Bis-(Methylthio)methane(CAS#1618-26-4)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 29309070 |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Dimethiomethane (also known as methyl sulfide) is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of dimethylthiomethane:
Quality:
- Appearance: Colorless liquid
- Odor: Has a strong smell of hydrogen sulfide
- Solubility: Soluble in many organic solvents such as ethanol and ether
Use:
- As a solvent: Dimethiomethane is an important organic solvent that can be used to dissolve and purify organic compounds.
- Chemical synthesis: It is often used as a reagent and intermediate in organic synthesis, and participates in some alkylation, oxidation, sulfidation and other reactions.
- Polymer materials: Dimethylthiomethane can also be used for crosslinking and modification of polymers.
Method:
- Dimethylthiomethane can be obtained by reacting methyl mercaptan with dimethyl mercaptan. In the reaction, sodium iodide or sodium bromide is usually used as a catalyst.
Safety Information:
- Dimethylthiomethane has a pungent odor and is also irritating to the eyes, skin and respiratory tract. Protective gloves, safety glasses and respiratory protection should be worn when in use.
- During storage and handling, contact with strong oxidizing agents and acids should be avoided to prevent dangerous chemical reactions.
- When burned, dimethylthiomethane produces toxic gases (e.g. sulphur dioxide) and should be used in a well-ventilated environment.
- When handling and disposing of waste, please follow the relevant local rules and regulations.