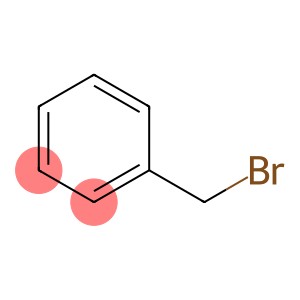
Benzyl bromide(CAS#100-39-0)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S39 – Wear eye / face protection. S2 – Keep out of the reach of children. |
| UN IDs | UN 1737 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | XS7965000 |
| FLUKA BRAND F CODES | 9-19-21 |
| TSCA | Yes |
| HS Code | 2903 99 80 |
| Hazard Class | 6.1 |
| Packing Group | II |
| Toxicity | dns-esc 1300 mmol/L ZKKOBW 92,177,78 |
Introduction
Benzyl bromide is an organic compound with the chemical formula C7H7Br. Here is some information about the properties, uses, preparation methods and safety of benzyl bromide:
Quality:
Benzyl bromide is a colorless liquid with a pungent odor at room temperature. Its density is 1.44g/mLat 20 °C, its boiling point is 198-199 °C(lit.), and its melting point is -3 °C. It can be dissolved in most organic solvents and is insoluble in water.
Use:
Benzyl bromide has a variety of uses. It is commonly used in organic synthesis as a reagent for reactions. It can be used in the preparation of esters, ethers, acid chlorides, ether ketones, and other organic compounds. In addition, benzyl bromide is also used as a chicken catalyst, light stabilizer, resin curing agent, and flame retardant for preparation.
Method:
Benzyl bromide can be prepared by the reaction of benzyl bromide and bromine under alkaline conditions. The specific step is to add bromine to benzyl bromide, and add alkali (such as sodium hydroxide) to obtain benzyl bromide after the reaction.
Safety Information:
Benzyl bromide is an organic compound that has certain toxicity. It has an irritating effect on the eyes, skin, and respiratory tract, so care should be taken when using personal protective equipment such as gloves, goggles, and face shields when touching. In addition, benzyl bromide also poses a burning hazard and should be avoided from contact with combustibles and kept away from open flames. When storing and handling benzyl bromide, follow the appropriate safe operating procedures and keep it in a safe place and avoid mixing it with other chemicals.