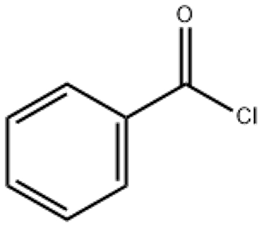
Benzoyl chloride CAS 98-88-4
Risk and Safety
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R43 – May cause sensitization by skin contact R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 1736 8/PG 2 |
| WGK Germany | 1 |
| RTECS | DM6600000 |
| TSCA | Yes |
| HS Code | 29310095 |
| Hazard Note | Corrosive |
| Hazard Class | 8 |
| Packing Group | II |
| Introduction | benzoyl chloride (CAS 98-88-4) also known as benzoyl chloride, benzoyl chloride, belonging to a kind of acid chloride. Pure colorless transparent flammable liquid, exposure to air smoke. Industrial products with light yellow, with a strong irritating odor. Vapor on the eye mucosa, skin and respiratory tract has a strong stimulating effect, by stimulating the eye mucosa and tear. Benzoyl chloride is an important intermediate for the preparation of dyes, fragrances, organic peroxides, pharmaceuticals and resins. It has also been used in photography and the production of artificial tannins, and has been used as a stimulant gas in chemical warfare. Figure 1 is the structural formula of benzoyl chloride |
| preparation method | in the laboratory, benzoyl chloride can be obtained by distilling benzoic acid and phosphorus pentachloride under anhydrous conditions. The industrial preparation method can be obtained by using thionyl chloride and benzaldehyde chloride. |
| hazard category | hazard category for benzoyl chloride: 8 |
| Use | benzoyl chloride is an intermediate of the herbicide oxazinone, and is also an intermediate of the insecticide benzenecapid, hydrazine inhibitor. benzoyl chloride is used as a raw material for organic synthesis, dyes and medicines, and as an initiator, dibenzoyl peroxide, tert-butyl peroxide, pesticide herbicide, etc. In terms of pesticides, is a new type of inducible insecticide isoxazole thiophos (isoxathon, Karphos) intermediates. It is also an important benzoylation and benzylation reagent. Most of benzoyl chloride is used to produce benzoyl peroxide, followed by the production of benzophenone, benzyl benzoate, benzyl cellulose and benzamide and other important chemical raw materials, benzoyl peroxide for the polymerization initiator of plastic monomer, polyester, epoxy, catalyst for acrylic resin production, self-coagulant for glass fiber material, crosslinking agent for silicone fluororubber, oil refining, flour bleaching, fiber decolorization, etc. In addition, benzoic acid can be reacted with benzoyl chloride to produce benzoic anhydride. The main use of benzoic anhydride is as an acylating agent, as a component of bleaching agent and flux, and also in the preparation of benzoyl peroxide. used as analytical reagents, also used in spices, organic synthesis |
| production method | 1. Toluene method raw materials toluene and chlorine in the light under the condition of reaction, side chain chlorination to produce α-trichlorotoluene, the latter in acidic medium hydrolysis to generate benzoyl chloride, and the release of hydrogen chloride gas (production of water absorption of HCl gas). 2. Benzoic acid and phosgene reaction. The benzoic acid is put into a photochemical pot, heated and melted, and phosgene is introduced at 140-150 ℃. The reaction tail gas contains hydrogen chloride and unreacted phosgene, which is treated with alkali and vented, the temperature at the end of the reaction was -2-3 °c, and the product was distilled under reduced pressure after the gas removal operation. Industrial products are yellowish transparent liquids. Purity ≥ 98%. Raw material consumption quota: benzoic acid 920kg/t, phosgene 1100kg/t, dimethylformamide 3kg/t, liquid alkali (30%)900kg/t. Now widely used in the industry of benzoic acid and benzylidene chloride reaction preparation. Benzoyl chloride can also be obtained by direct chlorination of benzaldehyde. There are several preparation methods. (1) The benzoic acid is heated and melted by phosgene method, and phosgene is introduced at 140~150 ℃, and a certain amount of phosgene is introduced to reach the end point. The phosgene is driven by nitrogen, and the tail gas is absorbed and destroyed, the final product was obtained by distillation under reduced pressure. (2) phosphorus trichloride method benzoic acid dissolved in toluene and other solvents, Phosphorus trichloride was added dropwise, and the reaction was carried out for several hours after dropping, the toluene was distilled off, and then the finished product was distilled off. (3) trichloromethylbenzene method to toluene side chain chlorination, and then hydrolysis product. |
Write your message here and send it to us



![1H-pyrrolo[2,3-b]pyridin-5-ol(CAS#98549-88-3)](https://www.xinchem.com/uploads/pyrrolo.gif)