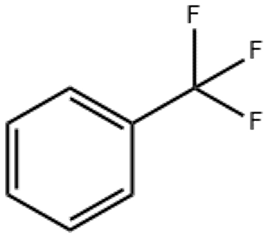
Benzotrifluoride(CAS# 98-08-8)
Risk and Safety
| Risk Codes | R45 – May cause cancer R46 – May cause heritable genetic damage R11 – Highly Flammable R36/38 – Irritating to eyes and skin. R48/23/24/25 - R65 – Harmful: May cause lung damage if swallowed R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R48/20/22 - R40 – Limited evidence of a carcinogenic effect R38 – Irritating to the skin R22 – Harmful if swallowed |
| Safety Description | S53 – Avoid exposure – obtain special instructions before use. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S23 – Do not breathe vapour. S16 – Keep away from sources of ignition. |
| UN IDs | UN 2338 3/PG 2 |
| WGK Germany | 3 |
| RTECS | XT9450000 |
| TSCA | Yes |
| HS Code | 29049090 |
| Hazard Note | Flammable/Corrosive |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 15000 mg/kg LD50 dermal Rat > 2000 mg/kg |
Information
| preparation | toluene trifluoride is an organic intermediate, which can be obtained from toluene as a raw material by chlorination and then fluorination. In the first step, chlorine, toluene and catalyst were mixed for chlorination reaction; The chlorination reaction temperature was 60 ℃ and the reaction pressure was 2Mpa; In the second step, hydrogen fluoride and catalyst were added to the nitrated mixture in the first step for fluorination reaction; The fluorination reaction temperature was 60 ℃ and the reaction pressure was 2MPa; In the third step, the mixture after the second fluorination reaction was subjected to rectification treatment to obtain trifluorotoluene. |
| uses | uses: for the manufacture of drugs, dyes, and used as curing agent, pesticides, etc. trifluoromethylbenzene is an important intermediate in fluorine chemistry, which can be used to prepare herbicides such as fluuron, fluralone, and pyrifluramine. It is also an important intermediate in medicine. intermediate of medicine and dye, solvent. And used as a curing agent and the manufacture of insulating oil. intermediates for organic synthesis and dyes, drugs, curing agents, accelerators, and for the manufacture of insulating oils. It can be used for the determination of the calorific value of fuel, the preparation of powder fire extinguishing agent, and the photodegradable plastic additive. |
| production method | 1. Derived from the interaction of ω,ω,ω-trichlorotoluene with anhydrous hydrogen fluoride. The molar ratio of ω,ω,ω-trichlorotoluene to anhydrous hydrogen fluoride is 1:3.88, and the reaction is carried out at a temperature of 80-104 ° C. Under a pressure of 1.67-1.77MPA for 2-3 hours. The yield was 72.1%. Because anhydrous hydrogen fluoride is cheap and easy to get, the equipment is easy to solve, no special steel, low cost, suitable for industrialization. Derived from the interaction of ω,ω,ω-toluene trifluoride with antimony trifluoride. The ω ω ω trifluorotoluene and antimony trifluoride are heated and distilled in a reaction pot, and the distillate is crude trifluoromethylbenzene. The mixture was washed with 5% hydrochloric acid, followed by 5% sodium hydroxide solution, and heated for distillation to collect the 80-105 °c fraction. The upper layer liquid was separated, and the lower layer liquid was dried with anhydrous calcium chloride and filtered to obtain trifluoromethylbenzene. The yield was 75%. This method consumes antimonide, the cost is higher, generally only in the laboratory conditions using more convenient. The preparation method is to use toluene as a raw material, first use chlorine gas in the presence of catalyst side chain chlorination to obtain α,α,α-trichlorotoluene, and then react with hydrogen fluoride to obtain the product. |
Write your message here and send it to us