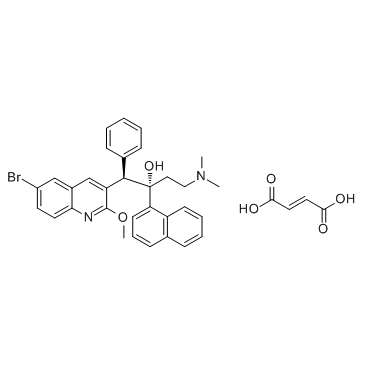
Bedaquiline (fumarate)(CAS#845533-86-0)
Bedaquiline (fumarate)(CAS#845533-86-0)
pharmachologic effect
Mechanism of action: Mainly through its quinoline group and dimethylaminogroup, it strongly interacts with Mycobacterium tuberculosis ATP synthase and binds to multiple sites in the transmembrane region, preventing the rotation of the C ring of ATP synthase in the transmembrane region, thereby blocking proton trafficking, and finally preventing the synthesis of ATP, so as to achieve the purpose of inhibiting and killing Mycobacterium tuberculosis.
Antibacterial characteristics: It is a representative diarylquinoline antimycobacterial drug, which has strong bactericidal activity against Mycobacterium tuberculosis, especially against drug-resistant tuberculosis bacteria, and is effective against sensitive, drug-resistant and dormant strains. It has a completely different mechanism of action from traditional anti-tuberculosis drugs such as isoniazid, rifampicin, pyrazinamide, etc., and there is no cross-resistance.
Clinical application
Indications: As part of combination therapy for the treatment of adult (≥18 years old) multidrug-resistant tuberculosis (MDR – TB). The US FDA has also approved it for the treatment of pediatric patients with multidrug-resistant tuberculosis in the lungs aged 5 years and older who weigh at least 15 kg. On July 20, 2023, China’s National Medical Products Administration approved its expansion for the treatment of MDR-TB in adolescents aged 12 to < 18 years weighing ≥ 30kg.
Dosage: Usually 400 mg once daily for the first 2 weeks; Thereafter, 200mg, 3 times a week.
CLINICAL STUDY: THE SIRTUIN 1 TRIAL SHOWED THAT PATIENTS TREATED WITH BEDAQUILINE HAD A FASTER CONVERSION RATE OF MYCOBACTERIUM TUBERCULOSIS IN SPUTUM, WITH A SPUTUM CULTURE CONVERSION RATE OF 58% IN BEDAQUILINE GROUP AND 32% IN PLACEBO GROUP AT 24 WEEKS OF TREATMENT.
Adverse effects
Common nausea, arthralgia, headache, etc., most of which are mild to moderate, are generally tolerated by patients, and symptoms are often relieved after stopping the drug.
This may result in a prolonged QT interval, with some patients having a QTcF greater than 500 ms.
Precautions
It must be used in combination with other antimycobacterial drugs and should not be used alone.
Patients should avoid alcohol, alcoholic beverages, and hepatotoxic drugs or herbal products.
Tell your doctor about other medications and other medical conditions you are taking before taking them.
Storage conditions
It should be protected from light, sealed, and stored below 30°C.