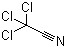
Barium sulfate CAS 13462-86-7
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S22 – Do not breathe dust. S24/25 – Avoid contact with skin and eyes. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| WGK Germany | - |
| RTECS | CR0600000 |
| TSCA | Yes |
| HS Code | 28332700 |
| Toxicity | LD50 orally in Rabbit: > 20000 mg/kg |
Introduction
Tasteless, non-toxic. Decomposition above 1600 ℃. Soluble in hot concentrated sulfuric acid, insoluble in water, organic and inorganic acids, caustic solution, soluble in hot sulfurous acid and hot concentrated sulfuric acid. The chemical properties are stable, and it is reduced to barium sulfide by heat with carbon. It does not change color when exposed to hydrogen sulfide or toxic gases in the air.
Write your message here and send it to us