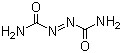Azodicarbonamide(CAS#123-77-3)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R42 – May cause sensitization by inhalation R44 – Risk of explosion if heated under confinement |
| Safety Description | S22 – Do not breathe dust. S24 – Avoid contact with skin. S37 – Wear suitable gloves. |
| UN IDs | UN 3242 4.1/PG 2 |
| WGK Germany | 1 |
| RTECS | LQ1040000 |
| HS Code | 29270000 |
| Hazard Class | 4.1 |
| Packing Group | II |
| Toxicity | LD50 oral in rat: > 6400mg/kg |
Introduction
Azodicarboxamide (N,N’-dimethyl-N,N’-dinitrosoglylamide) is a colorless crystalline solid with unique properties and a variety of applications.
Quality:
Azodicarboxamide is a colorless crystal at room temperature, soluble in acids, alkalis and organic solvents, and has good solubility.
It is susceptible to heat or blow and explode, and is classified as explosive.
Azodicarboxamide has strong oxidizing properties and can react violently with combustibles and easily oxidized substances.
Use:
Azodicarboxamide is widely used in the field of chemical synthesis and is an important reagent and intermediate in many organic synthesis reactions.
It is used as a raw material for dye pigments in the dye industry.
Method:
The preparation methods of azodicarbonamide are mainly as follows:
It is formed by the reaction of nitrous acid and dimethylurea.
It is produced by the reaction of soluble dimethylurea and dimethylurea initiated by nitric acid.
Safety Information:
Azodicarboxamide is highly explosive and should be kept away from ignition, friction, heat and other flammable substances.
Appropriate protective gloves, goggles, and masks should be worn when using azodicarbonamide.
Avoid contact with oxidants and combustibles during operation.
Azodicarbonamide should be stored in a sealed, cool, well-ventilated place away from direct sunlight.