azelastine hydrochloride(CAS#79307-93-0)
azelastine hydrochloride(CAS#79307-93-0) Introduction
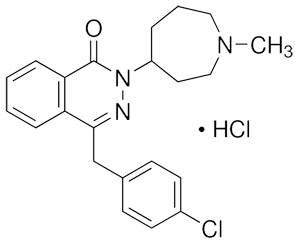
Azelastine hydrochloride
This product is (soil) 4-(4-chlorobenzyl)-2-(hexahydro-1-methyl-1 H-azazepine-4-yl)-l(2ff)-2,3-diazanaphthone hydrochloride. Calculated as a dry product, the content of C22H24C1N30•HCl shall not be less than 99.0%.
Characters
This product is white or off-white powder or crystalline powder; Odorless.
This product is slightly soluble in methanol, slightly soluble in water or ethanol, and soluble in glacial acetic acid.
Differential
take this product, add water to dissolve and dilute to make a solution containing about 30ug per 1ml, according to ultraviolet-visible spectrophotometry (General rule 0401), there is a maximum absorption at a wavelength of 286nm, and a minimum absorption at a wavelength of 264nm.
The infrared absorption spectrum of this product should be consistent with that of the reference substance (General rule 0402).
The aqueous solution of this product shows the reaction of chloride identification (1) (General rule 0301).
examine
optical rotation
take this product, weigh it accurately, add water to dissolve and quantitatively dilute it to make a solution containing about 5mg per love ml (if necessary, it can be dissolved by ultrasonic), and determine it according to law (General rule 0621), and the optical rotation is 0.01 ° to + 0.01 °.
acidity
take 50mg of this product, add 30ml of water to dissolve (if necessary, it can be dissolved by ultrasonic), and determine according to law (General rule 0631), the pH value should be 5.0 to 7.0.