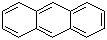
Anthracene(CAS#120-12-7)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R67 – Vapors may cause drowsiness and dizziness R36 – Irritating to the eyes R11 – Highly Flammable R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R65 – Harmful: May cause lung damage if swallowed R38 – Irritating to the skin R66 – Repeated exposure may cause skin dryness or cracking R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S24/25 – Avoid contact with skin and eyes. S16 – Keep away from sources of ignition. S9 – Keep container in a well-ventilated place. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label. S36 – Wear suitable protective clothing. |
| UN IDs | UN 3077 9/PG 3 |
| WGK Germany | 2 |
| RTECS | CA9350000 |
| TSCA | Yes |
| HS Code | 29029010 |
| Hazard Class | 9 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: > 16000 mg/kg |
Introduction
Anthracene is a polycyclic aromatic hydrocarbon. The following is an introduction to the properties, uses, preparation methods and safety information of anthracene:
Quality:
Anthracene is a dark yellow solid with a six-ring structure.
It has no special smell at room temperature.
It is almost insoluble in water but can be soluble in many organic solvents.
Use:
Anthracene is an important intermediate in the synthesis of many important organic compounds, such as dyes, fluorescent agents, pesticides, etc.
Method:
Commercially, anthracene is typically obtained by cracking coal tar in coal tar or in petrochemical processes.
In the laboratory, anthracene can be synthesized using catalysts through the interaction of benzene rings and aromatic hydrocarbons.
Safety Information:
Anthracene is toxic and should be avoided for long periods of time or in large quantities.
When in use, take the necessary protective measures, such as wearing gloves, face shields, and goggles, and ensure good ventilation.
Anthracene is a combustible substance, and fire and explosion prevention measures should be paid attention to, and it should be kept away from open flames and high temperatures.
Anthracene should not be discharged into the environment and the residue must be properly treated and disposed of.