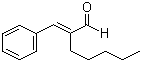
Amylcinnamaldehyde(CAS#122-40-7)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | UN 3082 9 / PGIII |
| WGK Germany | 2 |
| RTECS | GD6825000 |
| HS Code | 29122990 |
| Hazard Class | 9 |
| Packing Group | III |
| Toxicity | LD50 orl-rat: 3730 mg/kg FCTXAV 2,327,64 |
Amylcinnamaldehyde(Alpha-amylcinnamaldehyde)CAS#122-40-7
Quality:
Physical properties: Methylamyl cinnamaldehyde is a colorless liquid with a special aromatic odor.
Chemical properties: Methylamylcinnamaldehyde is a compound with strong activity. It reacts with oxygen to produce the corresponding acid. It can undergo acid-base neutralization reaction with alkalis such as sodium hydroxide to produce corresponding salts and water. Methylamylcinnamaldehyde can also react with other organic compounds such as esterification, addition and oxidation. In organic synthesis reactions, methylamyl cinnamaldehyde is also often used as an important intermediate.
Uses and synthesis methods
Methylamyl cinnamaldehyde (4-propyl-3,5-dimethylbenzaldehyde) is an organic compound, which is mainly used as a fragrance additive and fine chemical.
Methylamyl cinnamaldehyde is commonly used in perfume and flavor preparation because it has a unique aromatic smell that adds aroma and appeal to products.
The synthesis method of alphaamylcinnamaldehyde can be carried out by the following steps:
Using o-cresol as raw material, p-methylanisoles (p-methoxytoluene) are obtained by alkylation.
The aromatic hydroxylation reaction of p-methylanisoles was carried out to obtain 3,5-dimethylbenzyl alcohol.
3,5-dimethylbenzyl alcohol was subjected to acid-catalyzed oxidation to obtain methylamylcinnamaldehyde.
Through the above synthesis steps, high-purity alphaamyl cinnamaldehyde can be obtained.