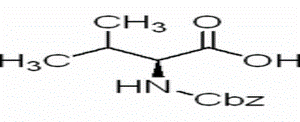
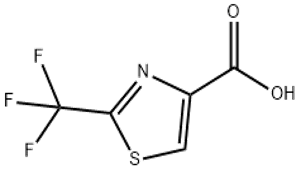
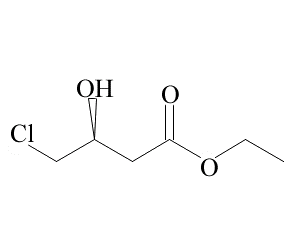
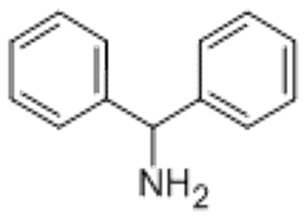Aminodiphenylmethane(CAS# 91-00-9)
| Risk Codes | R22 – Harmful if swallowed R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| UN IDs | 2810 |
| WGK Germany | 3 |
| RTECS | DA4407300 |
| FLUKA BRAND F CODES | 9-23 |
| HS Code | 29214990 |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
Dibenzylamine is an organic compound. It is a colorless, crystalline solid with a peculiar ammonia odor. The following is a detailed introduction to the properties, uses, preparation methods and safety information of diphenylmethylamine:
Quality:
- Appearance: Colorless crystalline solid
- Odor: Has a special smell of ammonia
- Solubility: Soluble in organic solvents such as ethers, alcohols and kerosene, almost insoluble in water
- Stability: Benzomethylamine is stable, but oxidation can occur under the action of strong oxidants
Use:
- Chemicals: Diphenylmethylamine is widely used in organic synthesis as a catalyst, reducing agent and coupling agent
- Dye industry: used in the synthesis of dyes
Method:
Dibenzomethylamine can be prepared by adding compounds such as aniline and benzaldehyde for reductive condensation reaction. The specific preparation method can be adjusted as needed, e.g. by selecting different catalysts and conditions.
Safety Information:
- Benzoamine is irritating to the skin, eyes, and respiratory tract and should be avoided.
- Wear appropriate protective equipment such as lab gloves, goggles, and lab clothing during handling.
- It should be operated in a well-ventilated area to avoid inhaling its vapors.
- Avoid contact with substances such as oxidants, strong acids or alkalis during storage to prevent dangerous reactions.
- In the event of an accident, remove the contaminants immediately, keep the airway open, and seek medical attention promptly.