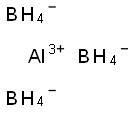
Aluminum borohydride(CAS#16962-07-5)
| UN IDs | 2870 |
| Hazard Class | 4.2 |
| Packing Group | I |
Introduction
Aluminium borohydride is an inorganic compound. It has the following properties:
1. Physical properties: Aluminum borohydride is a colorless solid, usually in powder form. It is very unstable at room temperature and must be stored and handled in a low temperature and inert gas environment.
2. Chemical properties: Aluminum borohydride can react with acids, alcohols, ketones and other compounds to form corresponding products. A violent reaction occurs in water to produce hydrogen and aluminic acid hydride.
The main uses of aluminium borohydride include:
1. As a reducing agent: Aluminum borohydride has strong reducing properties, and it is often used as a reducing agent in organic synthesis. It can reduce compounds such as aldehydes, ketones, etc., to the corresponding alcohols.
2. Scientific research use: Aluminum borohydride has important research value in the field of organic synthesis and catalysis, and can be used to synthesize new organic compounds and catalyze reactions.
There are generally two preparation methods for aluminum borohydride:
1. Reaction between aluminum hydroxide and trimethylboron: trimethylboron is dissolved in ethanol solution of aluminum hydroxide, hydrogen gas is introduced to obtain aluminum borohydride.
2. Reaction of alumina and dimethylborohydride: sodium dimethylborohydride and alumina are heated and reacted to obtain aluminum borohydride.
When using aluminium borohydride, the following safety information should be noted:
1. Aluminum borohydride has strong reducibility, and will react violently when in contact with water, acid and other substances, producing combustible gas and toxic gases. Protective glasses, gloves and protective clothing must be worn during operation.
2. Aluminum borohydride should be stored in a dry, sealed, and dark place, away from fire and flammable materials.
3. Invasion of the respiratory tract or skin can cause serious harm and must be avoided for inhalation and contact. In case of accidental contact, rinse immediately with plenty of water and seek medical attention.




![5-Fluoro-3-phenyl-2-[1-(9H-purin-6-ylamino)propyl]-4(3H)-quinazolinone(CAS#870281-82-6)](https://www.xinchem.com/uploads/quinazolinone.gif)