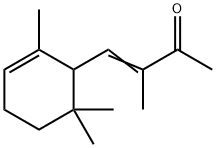
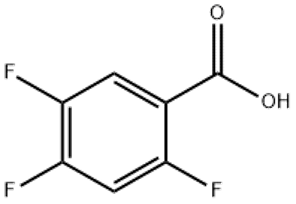
ALPHA-ISO-METHYLIONONE(CAS#127-51-5)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 43 – May cause sensitization by skin contact |
| Safety Description | S24/25 – Avoid contact with skin and eyes. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN1230 – class 3 – PG 2 – Methanol, solution |
| WGK Germany | 2 |
| Toxicity | GRAS(FEMA)。 |
Introduction
introduce
A mixture of methyl and ethyl isomethyl violet ketone and methyl and ethyl ortho methyl violet ketone.
nature
Alpha isomethylprednisolone is an organic compound. It is a purple crystalline solid. At room temperature, it has a unique aromatic odor.
Alpha isomethylprednisolone is an aromatic ketone. It is obtained from the methylation reaction of violet alcohol and is called isomethyl violet ketone. Its molecular structure contains an aromatic ring and a ketone group.
At room temperature, it is solid, but it can be dissolved by heating. It is insoluble in water, but can dissolve in some organic solvents such as ethanol and dichloromethane. It is sensitive to light and can undergo photolysis reaction under ultraviolet light irradiation.
In terms of chemical properties, α – isomethylprednisolone is a reactive compound. It can undergo reactions such as oxidation, reduction, addition, and substitution. It reacts with some electrophilic reagents to form addition products. It can be protonated in the presence of strong acids. It can also be converted into ketone acids through oxidation reactions.
Production method
The following is a common method for producing alpha isomethylprednisolone:
Initial material preparation: Prepare the initial materials, including Isobutyl ketone and Cyclohexanone. These two compounds are important precursors for the synthesis of α – isomethylprednisolone.
Reaction condition setting: Reacte isodecanone and cyclohexanol under appropriate conditions. A common reaction condition is to conduct the reaction under acidic conditions, and commonly used acid catalysts include sulfuric acid (H2SO4) and phosphoric acid (H3PO4).
Reaction steps: Mix a certain amount of isodecanone and cyclohexanol, and add an acid catalyst. Then, the reaction is carried out at an appropriate temperature, with a commonly used temperature range of 50-70 degrees Celsius. The reaction time is generally several hours to tens of hours.
Separation and purification: After the reaction is complete, the product is purified from the reaction mixture by distillation or other separation methods.
Crystallization and drying: Crystallize and dry the purified product to obtain the final alpha isomethylprednisolone product.