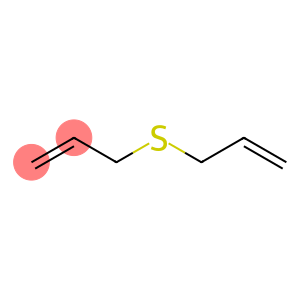
Allyl sulfide(CAS#592-88-1)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S37/39 – Wear suitable gloves and eye/face protection S23 – Do not breathe vapour. S16 – Keep away from sources of ignition. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 2 |
| RTECS | BC4900000 |
| TSCA | Yes |
| HS Code | 29309070 |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Allyl sulfide is an organic compound. It has the following properties:
Physical properties: Allyl sulfide is a colorless liquid with a strong pungent odor.
Chemical properties: Allyl sulfide is able to react with many compounds, especially reagents with electrophilicity, such as halogens, acids, etc. It can undergo polymerization reactions under certain conditions.
Main uses of allyl sulfide:
As an intermediate: Allyl sulfide can be used as an intermediate in organic synthesis and participate in a series of organic synthesis reactions, for example, it can be used to synthesize haloolefins and oxygen heterocyclic compounds.
There are several main methods for the preparation of allyl sulfide:
Hydrothiol substitution reaction: allyl sulfide can be formed by reactions such as allyl bromide and sodium hydrosulfide.
Allyl alcohol conversion reaction: prepared by the reaction of allyl alcohol and sulfuric acid.
From a safety perspective, allyl sulfide is an irritating substance that can cause irritation and damage in contact with the skin and eyes. Avoid direct contact with skin and eyes when using and maintain good ventilation conditions. Allyl sulfide is volatile and should be avoided for prolonged exposure to high concentrations of vapors or gases.