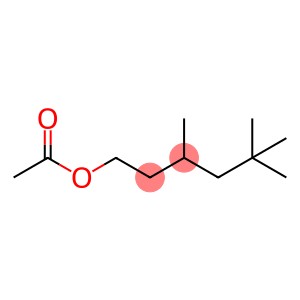
Aceticacidtrimethylester(CAS#58430-94-7)
Risk and Safety
| RTECS | AK0840000 |
| Toxicity | The acute oral LD50 in rats was reported as 4.25 g/kg (3.54-4.96 g/kg) (Moreno, 1973). The acute dermal LD50 in rabbits was reported as > 5 g/kg (Moreno, 1973). |
Aceticacidtrimethylester(CAS#58430-94-7) introduction
-3,5,5-trimethylhexyl acetate (also known as TMAH) is an organic compound. The following is an introduction to the nature, use, manufacturing method and safety information of TMAH:
Quality:
1. Appearance: TMAH is a colorless to light yellow liquid.
2. Solubility: TMAH is soluble in many organic solvents, such as alcohols, ethers and aromatics.
3. Stability: TMAH is relatively stable at room temperature and can separate pure products under distillation conditions.
Use:
1. Chemical synthesis: TMAH can be used as a catalyst, solvent and acid reagent in organic synthesis.
2. Semiconductor manufacturing: TMAH is commonly used as an etchant in semiconductor manufacturing to remove the oxide layer on silicon wafers.
Method:
The preparation of TMAH is usually carried out by the following steps:
1. Reaction of 3,5,5-trimethylhexanoic acid with ethanol alcohol to generate ethyl 3,5,5-trimethylhexanoic acid.
2. Ethyl 3,5,5-trimethylhexanoic acid is hydrolyzed with dilute stone alkali or acid to generate acetate-3,5,5-trimethylhexyl acetate.
Safety Information:
TMAH has an irritating effect on the skin and eyes, and prolonged contact with the skin or into the eyes should be avoided. Appropriate protective gear should be worn when in use.
Inhalation or ingestion of TMAH may cause poisoning, and should be kept away from inhaling its vapors or ingesting it by mistake.
When storing and using TMAH, follow appropriate safety procedures to ensure good ventilation and avoid contact with other toxic or flammable substances.
Caution should be exercised when using and handling chemical substances, and safety measures should be taken on a case-by-case basis.