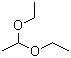
Acetal(CAS#105-57-7)
| Risk Codes | R11 – Highly Flammable R36/38 – Irritating to eyes and skin. |
| Safety Description | S9 – Keep container in a well-ventilated place. S16 – Keep away from sources of ignition. S33 – Take precautionary measures against static discharges. |
| UN IDs | UN 1088 3/PG 2 |
| WGK Germany | 2 |
| RTECS | AB2800000 |
| TSCA | Yes |
| HS Code | 29110000 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in rats: 4.57 g/kg (Smyth) |
Introduction
Acetal diethanol.
Properties: Acetal diethanol is a colorless to light yellow liquid with low vapor pressure. It is soluble in water, alcohols, and ether solvents and is a compound with good stability.
Uses: Acetal diethanol has excellent solubility, plasticity and wetting properties. It is often used as a solvent, wetting agent and lubricant.
Preparation method: acetal diethanol is generally prepared by epoxy compound cyclization reaction. Ethylene oxide is reacted with alcohol to obtain ethyl alcohol diethyl ether, which is then formed by acid-catalyzed hydrolysis to form acetal diethanol.
Safety information: Acetal diethanol is a low-toxicity compound, but it is still necessary to pay attention to safe use. Avoid contact with strong oxidants, strong acids and oxidants to prevent chemical reactions or dangerous accidents. Appropriate protective gloves, goggles, and overalls should be worn during use.