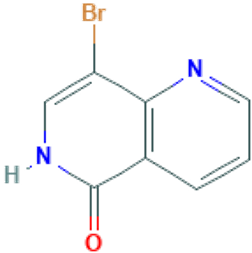
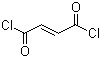
8-bromo-1 6-naphthyridin-5(6H)-one (CAS# 155057-97-9)
8-bromo-1, 6-naphythyridin-5 (6h)-one is an organic compound with the molecular formula C13H8BrNO, which is a powdery solid substance.
The properties of this compound are:
1. Appearance: 8-bromo-1, 6-naphythyridin-5 (6h)-one is white or light yellow crystal powder.
2. Melting Point: has a high melting point, about 206-210 ℃.
3. Solubility: It has good solubility in some organic solvents (such as chloroform, acetone and dimethyl sulfoxide).
It has many applications in the laboratory and industry:
1. Chemical reagents: can be used as reagents in organic synthesis reactions, such as applications in the synthesis of drugs and pesticides.
2. Photosensitive material: due to the special nature of its molecular structure, it can be used for the preparation of photosensitive materials.
3. Organic synthesis intermediates: can be used as intermediates for the synthesis of other compounds.
Regarding the preparation method, 8-bromo-1,6-naphthyridin-5(6h)-one can be prepared by the following steps:
1. First, 1,6-naphthoketone is reacted with hydrogen bromide. The reaction conditions may be carried out by heating in the presence of acetic acid.
2. The reaction product is 8-bromo -1,6-naphthoketone, further processing:
a. 8-bromo -1,6-naphthoketone is reacted with pyridine under acid catalysis.
B. Reaction conditions are Reflux reaction, usually in acetic acid.
c. The product obtained by the reaction is 8-bromo-1,6-naphthyridin-5(6h)-one.
Regarding safety information, 8-bromo-1,6-naphthyridin-5(6h)-one is an organic compound, so appropriate safety measures need to be taken:
1. Avoid contact with skin and eyes, such as contact, should immediately rinse with plenty of water.
2. Use should be carried out in a well-ventilated place to avoid inhaling its dust.
3. Should be stored away from the fire and oxidant.
4. Follow proper laboratory procedures and personal protective measures.