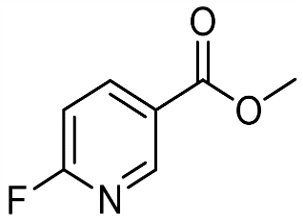
6-FLUORONICOTINIC ACID METHYL ESTER (CAS# 1427-06-1)
Risk and Safety
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R22 – Harmful if swallowed R41 – Risk of serious damage to eyes |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| WGK Germany | 3 |
| HS Code | 29333990 |
6-FLUORONICOTINIC ACID METHYL ESTER (CAS# 1427-06-1) Introduction
Methyl 6-fluorumicotinate, chemical formula C8H7FO3, is an organic compound. The following is a description of the properties, uses, preparation methods and safety information of methyl 6-fluorumicotinate:
Nature:
-Methyl 6-fluoronicotinate is a colorless liquid with a special smell.
-Its melting point is about -2°C, boiling point is about 164°C, and its relative density is about 1.36g/cm³.
-Methyl 6-fluoronicotinate is insoluble in water and soluble in some organic solvents such as ethanol and dichloromethane.
-It is a relatively stable compound, but it may degrade under light, heat, oxidants and strong acids.
Use:
-Methyl 6-fluoronicotinate can be used as an important intermediate in organic synthesis.
-It is commonly used in the preparation of pesticides, pharmaceuticals and dyes and other organic compounds.
Methyl 6-fluoronicotinate can also be used as a fluorination reagent, catalyst, solvent, etc. in organic synthesis reactions.
Preparation Method:
-The preparation of methyl 6-fluoricotinate is generally obtained by reacting ethyl acetate with hydrogen fluoride.
-The reaction conditions are generally required to be carried out at a low temperature, and a catalyst is used to promote the reaction.
Safety Information:
-Methyl 6-fluorumicotinate is an organic compound, and attention should be paid to the safe handling of chemicals.
-It is a flammable liquid and should be kept away from open flames and high temperature sources and stored in a closed container.
-Use with care to avoid contact with skin, eyes and respiratory tract.
-Use appropriate personal protective equipment such as gloves, safety glasses and protective masks when handling.
-In the event of a leak, appropriate emergency measures should be taken immediately to clean up and dispose of it.
Nature:
-Methyl 6-fluoronicotinate is a colorless liquid with a special smell.
-Its melting point is about -2°C, boiling point is about 164°C, and its relative density is about 1.36g/cm³.
-Methyl 6-fluoronicotinate is insoluble in water and soluble in some organic solvents such as ethanol and dichloromethane.
-It is a relatively stable compound, but it may degrade under light, heat, oxidants and strong acids.
Use:
-Methyl 6-fluoronicotinate can be used as an important intermediate in organic synthesis.
-It is commonly used in the preparation of pesticides, pharmaceuticals and dyes and other organic compounds.
Methyl 6-fluoronicotinate can also be used as a fluorination reagent, catalyst, solvent, etc. in organic synthesis reactions.
Preparation Method:
-The preparation of methyl 6-fluoricotinate is generally obtained by reacting ethyl acetate with hydrogen fluoride.
-The reaction conditions are generally required to be carried out at a low temperature, and a catalyst is used to promote the reaction.
Safety Information:
-Methyl 6-fluorumicotinate is an organic compound, and attention should be paid to the safe handling of chemicals.
-It is a flammable liquid and should be kept away from open flames and high temperature sources and stored in a closed container.
-Use with care to avoid contact with skin, eyes and respiratory tract.
-Use appropriate personal protective equipment such as gloves, safety glasses and protective masks when handling.
-In the event of a leak, appropriate emergency measures should be taken immediately to clean up and dispose of it.
Write your message here and send it to us


![1-(2 2-difluorobenzo[d][1 3]dioxol-5-yl)cyclopropanecarbonitrile(CAS# 862574-87-6)](https://www.xinchem.com/uploads/122difluorobenzod13dioxol5ylcyclopropanecarbonitrile.png)