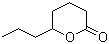
5-Octanolide(CAS#698-76-0)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R36/38 – Irritating to eyes and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S37 – Wear suitable gloves. |
| WGK Germany | 2 |
| RTECS | UQ1355500 |
| TSCA | Yes |
| HS Code | 29322090 |
| Toxicity | LD50 orl-rat: >5 g/kg FCTOD7 20,783,80 |
Introduction
δ-Octanolactone, also known as caprolactone, is an organic compound. It is a colorless to pale yellow liquid with a characteristic aroma of octanol. The following is an introduction to the properties, uses, preparation methods and safety information of δ-octanololide:
Quality:
- δ-Octanolactone is a volatile liquid that is soluble in water and many organic solvents.
- It is an unstable compound that is susceptible to polymerization and hydrolysis.
- It has low viscosity, low surface tension and good wetability.
Use:
- δ-Octanolactone is used in a wide range of applications, including plastics manufacturing, polymer synthesis, and surface coatings.
- It can be used as a component of solvents, catalysts and plasticizers.
- In the field of polymers, δ-octanol lactone can be used to prepare polycaprolactone (PCL) and other polymers.
- It can also be used in medical devices, coatings, adhesives, encapsulation materials, etc.
Method:
- δ-Octololide can be prepared by esterification of ε-caprolactone.
- The reaction is usually carried out under appropriate reaction conditions by reacting ε-caprolactone with an acid catalyst such as methanesulfonic acid.
- The preparation process requires control of reaction temperature and time to obtain a high-purity product.
Safety Information:
- It can be irritating to the skin, eyes, and respiratory tract and should be avoided when touched.
- During use and storage, it is necessary to maintain a well-ventilated environment and avoid fire sources and high temperatures.
- When disposing of waste, it should be handled and disposed of in accordance with local regulations.


![4-[(4-Hydroxy-2-pyrimidinyl)amino]benzonitrile(CAS# 189956-45-4)](https://www.xinchem.com/uploads/44Hydroxy2pyrimidinylaminobenzonitrile.png)